Increased flexibility decreases antifreeze protein activity
- PMID: 20936690
- PMCID: PMC3009403
- DOI: 10.1002/pro.516
Increased flexibility decreases antifreeze protein activity
Abstract
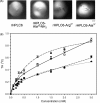
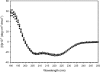
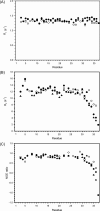
Antifreeze proteins protect several cold-blooded organisms from subzero environments by preventing death from freezing. The Type I antifreeze protein (AFP) isoform from Pseudopleuronectes americanus, named HPLC6, is a 37-residue protein that is a single α-helix. Mutational analysis of the protein showed that its alanine-rich face is important for binding to and inhibiting the growth of macromolecular ice. Almost all structural studies of HPLC6 involve the use of chemically synthesized protein as it requires a native N-terminal aspartate and an amidated C-terminus for full activity. Here, we examine the role of C-terminal amide and C-terminal arginine side chain in the activity, structure, and dynamics of nonamidated Arg37 HPLC6, nonamidated HPLC6 Ala37, amidated HPLC6 Ala37, and fully native HPLC6 using a recombinant bacterial system. The thermal hysteresis (TH) activities of the nonamidated mutants are 35% lower compared with amidated proteins, but analysis of the NMR data and circular dichroism spectra shows that they are all still α-helical. Relaxation data from the two nonamidated mutants indicate that the C-terminal residues are considerably more flexible than the rest of the protein because of the loss of the amide group, whereas the amidated Ala37 mutant has a C-terminus that is as rigid as the wild-type protein and has high TH activity. We propose that an increase in flexibility of the AFP causes it to lose activity because its dynamic nature prevents it from binding strongly to the ice surface.
Copyright © 2010 The Protein Society.
Figures
References
-
- Scholander PF, van Dam L, Kanwisher J, Hammel T, Gordon MS. Supercooling and osmoregulation in arctic fish. J Cell Comp Physiol. 1957;49:5–24.
-
- DeVries AL, Wohlschlag DE. Freezing resistance in some Antarctic fishes. Science. 1969;163:1073–1075. - PubMed
-
- Graham LA, Liou YC, Walker VK, Davies PL. Hyperactive antifreeze protein from beetles. Nature. 1997;388:727–728. - PubMed
-
- Xu H, Griffith M, Patten CL, Glick BR. Isolation and characterization of an antifreeze protein with ice nucleation activity from the plant growth promoting rhizobacterium Pseudomonas putida GR12-2. Can J Micro. 1998;44:64–73.
-
- Hoshino T, Kiriaki M, Ohgiya S, Fujiwara M, Kondo H, Nishimiya Y, Yumoto I, Tsuda S. Antifreeze proteins from snow mold fungi. Can J Botany. 2003;81:1175–1181.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

