Characterizing the contribution of stem/progenitor cells to tumorigenesis in the Pten-/-TP53-/- prostate cancer model
- PMID: 20936707
- PMCID: PMC7454246
- DOI: 10.1002/stem.538
Characterizing the contribution of stem/progenitor cells to tumorigenesis in the Pten-/-TP53-/- prostate cancer model
Abstract
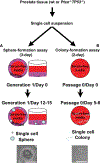
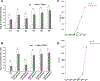
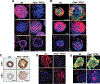
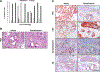
Loss of PTEN is one of the most common mutations in prostate cancer, and loss of wild-type TP53 is associated with prostate cancer progression and castrate resistance. Modeling prostate cancer in the mouse has shown that while Pten deletion in prostate epithelial cells leads to adenocarcinoma, combined loss of Pten and TP53 results in rapidly developing disease with greater tumor burden and early death. TP53 contributes significantly to the regulation of stem cell self-renewal, and we hypothesized that loss of Pten/TP53 would result in measurable changes in prostate cancer stem/progenitor cell properties. Clonogenic assays that isolate progenitor function in primary prostate epithelial cells were used to measure self-renewal, differentiation, and tumorigenic potential. Pten/TP53 null as compared with wild-type protospheres showed increased self-renewal activity and modified lineage commitment. Orthotopic transplantation of Pten/TP53 null cells derived from protospheres produced invasive Prostatic Intraepithelial Neoplasia (PIN)/adenocarcinoma, recapitulating the pathology seen in primary tumors. Pten/TP53 null progenitors relative to wild type also demonstrated increased dependence on the AKT/mammalian target of rapamycin complex 1 (mTORC1) and androgen receptor (AR) pathways for clonogenic and tumorigenic growth. These data demonstrate roles for Pten/TP53 in prostate epithelial stem/progenitor cell function, and moreover, as seen in patients with castrate-resistant prostate cancer, suggest for the involvement of an AR-dependent axis in the clonogenic expansion of prostate cancer stem cells.
Conflict of interest statement
D
The authors indicate no potential conflicts of interest.
Figures


References
-
- Roudier MP, True LD, Higano CS et al. Phenotypic heterogeneity of end-stage prostate carcinoma metastatic to bone. Hum Pathol 2003;34: 646–653. - PubMed
-
- Lapointe J, Li C, Giacomini CP et al. Genomic profiling reveals alternative genetic pathways of prostate tumorigenesis. Cancer Res 2007; 67:8504–8510. - PubMed
-
- Sircar K, Yoshimoto M, Monzon FA et al. PTEN genomic deletion is associated with p-Akt and AR signalling in poorer outcome, hormone refractory prostate cancer. J Pathol 2009;218:505–513. - PubMed
-
- Agell L, Hernandez S, de Muga S et al. KLF6 and TP53 mutations are a rare event in prostate cancer: Distinguishing between Taq polymerase artifacts and true mutations. Mod Pathol 2008;21:1470–1478. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

