The effects of midodrine on the natriuretic response to furosemide in cirrhotics with ascites
- PMID: 20937051
- PMCID: PMC3154138
- DOI: 10.1111/j.1365-2036.2010.04426.x
The effects of midodrine on the natriuretic response to furosemide in cirrhotics with ascites
Abstract
Background: Resistance to loop diuretics is common in patients with ascites. Diminished glomerular filtration rate (GFR) is thought to mediate resistance to loop diuretics. Midodrine, a commonly used alpha-1 agonist, has been shown to improve GFR in non-azotemic patients with cirrhosis.
Aim: To conduct a randomized, double-blind, placebo-controlled, cross-over study to test the hypothesis that midodrine significantly increases natriuretic response of IV furosemide in non-azotemic cirrhotics with ascites.
Methods: All subjects participated in both phases, which were (i) furosemide IV infusion + oral midodrine 15 mg administered 30 min before furosemide (ii) furosemide IV infusion + oral placebo administered 30 min before furosemide. Primary outcomes were 6-h urine sodium excretion and 6-h total urine volume.
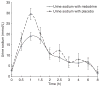
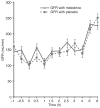
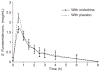
Results: A total of 15 patients (men: 8; age: 52.7 ± 7.6 years; serum creatinine: 1.06 ± 0.2 mg/dL) were studied. Total 6-h urine sodium excretion was 109 ± 42 mmol in the furosemide + midodrine treatment phase and was not significantly different from that in the furosemide + placebo treatment phase (126 ± 69 mmol, P = 0.6). Similarly, mean 6-h total urine volume was not significantly different between two groups (1770 ± 262 mL vs. 1962 ± 170 mL, P = 0.25).
Conclusions: Oral midodrine does not increase the natriuretic response to furosemide in non-azotemic cirrhotic patients with ascites. Orally administered midodrine does not increase natriuretic response to furosemide in non-azotemic cirrhotic patients with ascites.
© 2010 Blackwell Publishing Ltd.
Conflict of interest statement
Figures


Comment in
-
Midodrine and furosemide-induced natriuresis in cirrhotics with ascites.Aliment Pharmacol Ther. 2011 Feb;33(3):415-6. doi: 10.1111/j.1365-2036.2010.04536.x. Aliment Pharmacol Ther. 2011. PMID: 21198702 No abstract available.
Similar articles
-
Effects of a 7-day treatment with midodrine in non-azotemic cirrhotic patients with and without ascites.J Hepatol. 2007 Feb;46(2):213-21. doi: 10.1016/j.jhep.2006.09.012. Epub 2006 Nov 10. J Hepatol. 2007. PMID: 17156883 Clinical Trial.
-
Acute effects of the oral administration of midodrine, an alpha-adrenergic agonist, on renal hemodynamics and renal function in cirrhotic patients with ascites.Hepatology. 1998 Oct;28(4):937-43. doi: 10.1002/hep.510280407. Hepatology. 1998. PMID: 9755229
-
Clinical study on the therapeutic role of midodrine in non azotemic cirrhotic patients with tense ascites: a double-blind, placebo-controlled, randomized trial.Hepatogastroenterology. 2014 Oct;61(135):1915-24. Hepatogastroenterology. 2014. PMID: 25713888 Clinical Trial.
-
The significance of the furosemide test for predicting ascites control by diuretics in cirrhotics: a comparison with volume expansion and octreotide infusion.Dig Dis Sci. 2006 Nov;51(11):1992-7. doi: 10.1007/s10620-005-9072-2. Epub 2006 Oct 20. Dig Dis Sci. 2006. PMID: 17053959
-
Regulation of renal sodium and water excretion in the nephrotic syndrome and cirrhosis of the liver.Dan Med Bull. 1997 Apr;44(2):191-207. Dan Med Bull. 1997. PMID: 9151012 Review.
Cited by
-
Refractory ascites-the contemporary view on pathogenesis and therapy.PeerJ. 2019 Oct 15;7:e7855. doi: 10.7717/peerj.7855. eCollection 2019. PeerJ. 2019. PMID: 31637125 Free PMC article.
-
Midodrine and Weekly Albumin Therapy in Patients With Cirrhosis and Diuretic Intractable or Recurrent Ascites: A Case-Control Study.Cureus. 2025 Jan 6;17(1):e76988. doi: 10.7759/cureus.76988. eCollection 2025 Jan. Cureus. 2025. PMID: 39912014 Free PMC article.
-
Treatment for ascites in adults with decompensated liver cirrhosis: a network meta-analysis.Cochrane Database Syst Rev. 2020 Jan 16;1(1):CD013123. doi: 10.1002/14651858.CD013123.pub2. Cochrane Database Syst Rev. 2020. PMID: 31978257 Free PMC article.
-
What is the Role of Midodrine in Patients with Decompensated Cirrhosis?Gastroenterol Hepatol (N Y). 2011 Feb;7(2):134-6. Gastroenterol Hepatol (N Y). 2011. PMID: 21475424 Free PMC article. No abstract available.
-
Midodrine in Liver Cirrhosis With Ascites: A Systematic Review and Meta-Analysis.Cureus. 2022 Jul 30;14(7):e27483. doi: 10.7759/cureus.27483. eCollection 2022 Jul. Cureus. 2022. PMID: 36060403 Free PMC article. Review.
References
-
- Hoyert DL, Heron MP, Murphy SL, et al. Deaths: final data for 2006. Natl Vital Stat Rep. 2009;57:1–135. - PubMed
-
- Sherlock S, Dooley J. Ascites. In: Sherlock S, editor. Diseases of the Liver and Biliary System. 11. London: Blackwell; 2002. p. 139.
-
- Ruhl CE, Sayer B, Byrd-Holt DD, et al. Costs of digestive diseases. In: Everhart J, editor. The Burden of Digestive Diseases in the United States. Washington, DC: US Department of Health and Human Services, Public Health Service, National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases. US Government Printing Office NIH Publication No. 09-6443; 2008. pp. 137–46.
-
- Runyon BA. Historical aspects of treatment of patients with cirrhosis and ascites. Semin Liver Dis. 1997;17:163–73. - PubMed
-
- Runyon BA. Management of adult patients with ascites caused by cirrhosis. Hepatology. 1998;27:264–72. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
