Interaction of mumps virus V protein variants with STAT1-STAT2 heterodimer: experimental and theoretical studies
- PMID: 20937132
- PMCID: PMC2958915
- DOI: 10.1186/1743-422X-7-263
Interaction of mumps virus V protein variants with STAT1-STAT2 heterodimer: experimental and theoretical studies
Abstract
Background: Mumps virus V protein has the ability to inhibit the interferon-mediated antiviral response by inducing degradation of STAT proteins. Two virus variants purified from Urabe AM9 mumps virus vaccine differ in their replication and transcription efficiency in cells primed with interferon. Virus susceptibility to IFN was associated with insertion of a non-coded glycine at position 156 in the V protein (VGly) of one virus variant, whereas resistance to IFN was associated with preservation of wild-type phenotype in the V protein (VWT) of the other variant.
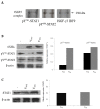
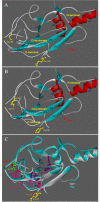
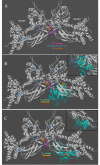
Results: VWT and VGly variants of mumps virus were cloned and sequenced from Urabe AM9 vaccine strain. VGly differs from VWT protein because it possesses an amino acid change Gln103Pro (Pro103) and the Gly156 insertion. The effect of V protein variants on components of the interferon-stimulated gene factor 3 (ISGF3), STAT1 and STAT2 proteins were experimentally tested in cervical carcinoma cell lines. Expression of VWT protein decreased STAT1 phosphorylation, whereas VGly had no inhibitory effect on either STAT1 or STAT2 phosphorylation. For theoretical analysis of the interaction between V proteins and STAT proteins, 3D structural models of VWT and VGly were predicted by comparing with simian virus 5 (SV5) V protein structure in complex with STAT1-STAT2 heterodimer. In silico analysis showed that VWT-STAT1-STAT2 complex occurs through the V protein Trp-motif (W174, W178, W189) and Glu95 residue close to the Arg409 and Lys415 of the nuclear localization signal (NLS) of STAT2, leaving exposed STAT1 Lys residues (K85, K87, K296, K413, K525, K679, K685), which are susceptible to proteasome degradation. In contrast, the interaction between VGly and STAT1-STAT2 heterodimer occurs in a region far from the NLS of STAT2 without blocking of Lys residues in both STAT1 and STAT2.
Conclusions: Our results suggest that VWT protein of Urabe AM9 strain of mumps virus may be more efficient than VGly to inactivate both the IFN signaling pathway and antiviral response due to differences in their finest molecular interaction with STAT proteins.
Figures
Similar articles
-
Modulation of apoptosis by V protein mumps virus.Virol J. 2011 May 13;8:224. doi: 10.1186/1743-422X-8-224. Virol J. 2011. PMID: 21569530 Free PMC article.
-
Differential sensitivity to interferon influences the replication and transcription of Urabe AM9 mumps virus variants in nerve cells.Microbes Infect. 2007 Jun;9(7):864-72. doi: 10.1016/j.micinf.2007.03.005. Epub 2007 Mar 15. Microbes Infect. 2007. PMID: 17533145
-
Degradation of STAT1 and STAT2 by the V proteins of simian virus 5 and human parainfluenza virus type 2, respectively: consequences for virus replication in the presence of alpha/beta and gamma interferons.J Virol. 2002 Mar;76(5):2159-67. doi: 10.1128/jvi.76.5.2159-2167.2002. J Virol. 2002. PMID: 11836393 Free PMC article.
-
A Positive Feedback Amplifier Circuit That Regulates Interferon (IFN)-Stimulated Gene Expression and Controls Type I and Type II IFN Responses.Front Immunol. 2018 May 28;9:1135. doi: 10.3389/fimmu.2018.01135. eCollection 2018. Front Immunol. 2018. PMID: 29892288 Free PMC article. Review.
-
The unique role of STAT2 in constitutive and IFN-induced transcription and antiviral responses.Cytokine Growth Factor Rev. 2016 Jun;29:71-81. doi: 10.1016/j.cytogfr.2016.02.010. Epub 2016 Mar 18. Cytokine Growth Factor Rev. 2016. PMID: 27053489 Review.
Cited by
-
Modulation of apoptosis by V protein mumps virus.Virol J. 2011 May 13;8:224. doi: 10.1186/1743-422X-8-224. Virol J. 2011. PMID: 21569530 Free PMC article.
-
The V protein of mumps virus plays a critical role in pathogenesis.J Virol. 2012 Feb;86(3):1768-76. doi: 10.1128/JVI.06019-11. Epub 2011 Nov 16. J Virol. 2012. PMID: 22090137 Free PMC article.
-
Advancements in the genomic feature of Newcastle disease virus and the multifaceted roles of non-structural proteins V/W in viral replication and pathogenesis.Poult Sci. 2025 Jul 3;104(10):105527. doi: 10.1016/j.psj.2025.105527. Online ahead of print. Poult Sci. 2025. PMID: 40639003 Free PMC article. Review.
-
Mumps virus pathogenesis: Insights and knowledge gaps.Hum Vaccin Immunother. 2016 Dec;12(12):3110-3112. doi: 10.1080/21645515.2016.1210745. Epub 2016 Jul 25. Hum Vaccin Immunother. 2016. PMID: 27455055 Free PMC article.
-
Identification of chikungunya virus interacting proteins in mammalian cells.J Biosci. 2014 Jun;39(3):389-99. doi: 10.1007/s12038-014-9436-x. J Biosci. 2014. PMID: 24845503
References
-
- Goodbourn S, Didcock L, Randall RE. Interferons: cell signaling, immune modulation, antiviral response and virus countermeasures. J Gen Virol. 2000;81(10):2341–2364. - PubMed
-
- de Weerd NA, Samarajiwa SA, Hertzog PJ. Type I interferon receptors: biochemistry and biological functions. J Biol Cel. 2007;282(28):20053–20057. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

