Context-dependent role of angiopoietin-1 inhibition in the suppression of angiogenesis and tumor growth: implications for AMG 386, an angiopoietin-1/2-neutralizing peptibody
- PMID: 20937592
- PMCID: PMC4430860
- DOI: 10.1158/1535-7163.MCT-10-0213
Context-dependent role of angiopoietin-1 inhibition in the suppression of angiogenesis and tumor growth: implications for AMG 386, an angiopoietin-1/2-neutralizing peptibody
Abstract
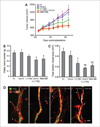
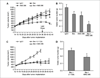
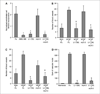
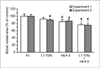
AMG 386 is an investigational first-in-class peptide-Fc fusion protein (peptibody) that inhibits angiogenesis by preventing the interaction of angiopoietin-1 (Ang1) and Ang2 with their receptor, Tie2. Although the therapeutic value of blocking Ang2 has been shown in several models of tumorigenesis and angiogenesis, the potential benefit of Ang1 antagonism is less clear. To investigate the consequences of Ang1 neutralization, we have developed potent and selective peptibodies that inhibit the interaction between Ang1 and its receptor, Tie2. Although selective Ang1 antagonism has no independent effect in models of angiogenesis-associated diseases (cancer and diabetic retinopathy), it induces ovarian atrophy in normal juvenile rats and inhibits ovarian follicular angiogenesis in a hormone-induced ovulation model. Surprisingly, the activity of Ang1 inhibitors seems to be unmasked in some disease models when combined with Ang2 inhibitors, even in the context of concurrent vascular endothelial growth factor inhibition. Dual inhibition of Ang1 and Ang2 using AMG 386 or a combination of Ang1- and Ang2-selective peptibodies cooperatively suppresses tumor xenograft growth and ovarian follicular angiogenesis; however, Ang1 inhibition fails to augment the suppressive effect of Ang2 inhibition on tumor endothelial cell proliferation, corneal angiogenesis, and oxygen-induced retinal angiogenesis. In no case was Ang1 inhibition shown to (a) confer superior activity to Ang2 inhibition or dual Ang1/2 inhibition or (b) antagonize the efficacy of Ang2 inhibition. These results imply that Ang1 plays a context-dependent role in promoting postnatal angiogenesis and that dual Ang1/2 inhibition is superior to selective Ang2 inhibition for suppression of angiogenesis in some postnatal settings.
Conflict of interest statement
All authors (with the exception of B.L. Falcón and D.M. McDonald) are current or former employees of Amgen, Inc.
Figures

References
-
- Perry BN, Arbiser JL. The duality of angiogenesis: implications for therapy of human disease. J Invest Dermatol. 2006;126:2160–2166. - PubMed
-
- van Cruijsen H, van der Veldt A, Hoekman K. Tyrosine kinase inhibitors of VEGF receptors: clinical issues and remaining questions. Front Biosci. 2009;14:2248–2268. - PubMed
-
- Rosenfeld PJ, Brown DM, Heier JS, et al. Ranibizumab for neovascular age-related macular degeneration. N Engl J Med. 2006;355:1419–1431. - PubMed
-
- Gragoudas ES, Adamis AP, Cunningham ET, Feinsod M, Guyer DR. Pegaptanib for neovascular age-related macular degeneration. N Engl J Med. 2004;351:2805–2816. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

