Maytansine and cellular metabolites of antibody-maytansinoid conjugates strongly suppress microtubule dynamics by binding to microtubules
- PMID: 20937594
- PMCID: PMC2954514
- DOI: 10.1158/1535-7163.MCT-10-0644
Maytansine and cellular metabolites of antibody-maytansinoid conjugates strongly suppress microtubule dynamics by binding to microtubules
Abstract
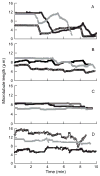
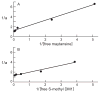
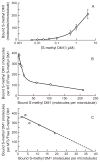
Maytansine is a potent microtubule-targeted compound that induces mitotic arrest and kills tumor cells at subnanomolar concentrations. However, its side effects and lack of tumor specificity have prevented successful clinical use. Recently, antibody-conjugated maytansine derivatives have been developed to overcome these drawbacks. Several conjugates show promising early clinical results. We evaluated the effects on microtubule polymerization and dynamic instability of maytansine and two cellular metabolites (S-methyl-DM1 and S-methyl-DM4) of antibody-maytansinoid conjugates that are potent in cells at picomolar levels and that are active in tumor-bearing mice. Although S-methyl-DM1 and S-methyl-DM4 inhibited polymerization more weakly than maytansine, at 100 nmol/L they suppressed dynamic instability more strongly than maytansine (by 84% and 73%, respectively, compared with 45% for maytansine). However, unlike maytansine, S-methyl-DM1 and S-methyl-DM4 induced tubulin aggregates detectable by electron microscopy at concentrations ≥2 μmol/L, with S-methyl-DM4 showing more extensive aggregate formation than S-methyl-DM1. Both maytansine and S-methyl-DM1 bound to tubulin with similar K(D) values (0.86 ± 0.2 and 0.93 ± 0.2 μmol/L, respectively). Tritiated S-methyl-DM1 bound to 37 high-affinity sites per microtubule (K(D), 0.1 ± 0.05 μmol/L). Thus, S-methyl-DM1 binds to high-affinity sites on microtubules 20-fold more strongly than vinblastine. The high-affinity binding is likely at microtubule ends and is responsible for suppression of microtubule dynamic instability. Also, at higher concentrations, S-methyl-DM1 showed low-affinity binding either to a larger number of sites on microtubules or to sedimentable tubulin aggregates. Overall, the maytansine derivatives that result from cellular metabolism of the antibody conjugates are themselves potent microtubule poisons, interacting with microtubules as effectively as or more effectively than the parent molecule.
Figures

References
-
- Kupchan SM, Komoda Y, Branfman AR, et al. The maytansinoids. Isolation, structural elucidation, and chemical interrelation of novel ansa macrolides. J Org Chem. 1977;42:2349–57. - PubMed
-
- Kupchan SM, Komoda Y, Court WA, et al. Maytansine, a novel antileukemic ansa macrolide from Maytenus ovatus. J Am Chem Soc. 1972;94:1354–6. - PubMed
-
- Remillard S, Rebhun LI, Howie GA, Kupchan SM. Antimitotic activity of the potent tumor inhibitor maytansine. Science. 1975;189:1002–5. - PubMed
-
- Mandelbaum-Shavit F, Wolpert-DeFilippes MK, Johns DG. Binding of maytansine to rat brain tubulin. Biochem Biophys Res Commun. 1976;72:47–54. - PubMed
-
- Bhattacharyya B, Wolff J. Maytansine binding to the vinblastine sites of tubulin. FEBS Lett. 1977;75:159–62. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

