Determinants of postinfarction ventricular tachycardia
- PMID: 20937722
- PMCID: PMC3091263
- DOI: 10.1161/CIRCEP.110.945295
Determinants of postinfarction ventricular tachycardia
Abstract
Background: Structural factors contributing to the development of postinfarction ventricular tachycardia (VT) are unclear. The purpose of this study was to analyze infarct architecture and electrogram characteristics in patients with and without inducible VT and to identify correlates of postinfarction VT.
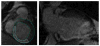
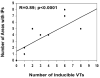
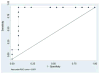
Methods and results: Twenty-four postinfarction patients (median age, 64 [53, 70] years) were referred for radiofrequency catheter ablation of VT (n = 12) or frequent symptomatic premature ventricular contractions (PVCs) (n = 12). Delayed-enhanced (DE) MRI was obtained before ablation. Electroanatomical mapping was performed and scar area and electrogram characteristics of the scar tissue compared in patients with and without inducible VT. The median ejection fraction in patients with and without inducible VT was 27% (22%, 43%) and 43% (40%, 47%), respectively (P = 0.085). Subendocardial infarct area determined by DE-MRI was larger in patients with inducible VT (43 [38, 62] cm(2)) than in those with noninducible VT (8 [4, 11] cm(2); P = 0.002), and unipolar and bipolar voltages on electroanatomical maps were significantly lower in patients with inducible VT (both P<0.05). An infarct volume of >14% identified 11 of 12 patients with inducible VT (area under the curve, 0.94; P = 0.007). On electroanatomical mapping, distinct sites with isolated potentials (IPs) were more prevalent in patients with inducible VT than in those without (13.2% versus 1.1% of points within scar; P < 0.001). The number of inducible VTs correlated with the number of distinct sites with IPs (R = 0.87; P<0.0001).
Conclusions: Scar tissue in postinfarction patients with inducible VT shows quantitative and qualitative differences from scars in patients without inducible VT. Scar size and IPs are correlated with VT inducibility.
Conflict of interest statement
Figures



References
-
- Kaplinsky E, Horowitz A, Neufeld HN. Ventricular reentry and automaticity in myocardial infarction. Effect of size of injury. Chest. 1978;74:66–71. - PubMed
-
- Califf RM, Burks JM, Behar VS, Margolis JR, Wagner GS. Relationships among ventricular arrhythmias, coronary artery disease, and angiographic and electrocardiographic indicators of myocardial fibrosis. Circulation. 1978;57:725–32. - PubMed
-
- Bolick DR, Hackel DB, Reimer KA, Ideker RE. Quantitative analysis of myocardial infarct structure in patients with ventricular tachycardia. Circulation. 1986;74:1266–79. - PubMed
-
- Simonetti OP, Kim RJ, Fieno DS, Hillenbrand HB, Wu E, Bundy JM, Finn JP, Judd RM. An improved MR imaging technique for the visualization of myocardial infarction. Radiology. 2001;218:215–23. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

