Anaphase-promoting complex/cyclosome participates in the acute response to protein-damaging stress
- PMID: 20937767
- PMCID: PMC3004273
- DOI: 10.1128/MCB.01506-09
Anaphase-promoting complex/cyclosome participates in the acute response to protein-damaging stress
Abstract
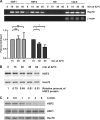
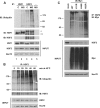
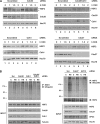
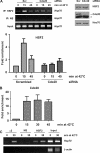
The ubiquitin E3 ligase anaphase-promoting complex/cyclosome (APC/C) drives degradation of cell cycle regulators in cycling cells by associating with the coactivators Cdc20 and Cdh1. Although a plethora of APC/C substrates have been identified, only a few transcriptional regulators are described as direct targets of APC/C-dependent ubiquitination. Here we show that APC/C, through substrate recognition by both Cdc20 and Cdh1, mediates ubiquitination and degradation of heat shock factor 2 (HSF2), a transcription factor that binds to the Hsp70 promoter. The interaction between HSF2 and the APC/C subunit Cdc27 and coactivator Cdc20 is enhanced by moderate heat stress, and the degradation of HSF2 is induced during the acute phase of the heat shock response, leading to clearance of HSF2 from the Hsp70 promoter. Remarkably, Cdc20 and the proteasome 20S core α2 subunit are recruited to the Hsp70 promoter in a heat shock-inducible manner. Moreover, the heat shock-induced expression of Hsp70 is increased when Cdc20 is silenced by a specific small interfering RNA (siRNA). Our results provide the first evidence for participation of APC/C in the acute response to protein-damaging stress.
Figures



References
-
- Abravaya, K., B. Phillips, and R. I. Morimoto. 1991. Attenuation of the heat shock response in HeLa cells is mediated by the release of bound heat shock transcription factor and is modulated by changes in growth and in heat shock temperatures. Genes Dev. 5:2117-2127. - PubMed
-
- Adhikary, S., F. Marinoni, A. Hock, E. Hulleman, N. Popov, R. Beier, S. Bernard, M. Quarto, M. Capra, S. Goettig, U. Kogel, M. Scheffner, K. Helin, and M. Eilers. 2005. The ubiquitin ligase HectH9 regulates transcriptional activation by Myc and is essential for tumor cell proliferation. Cell 123:409-421. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous
