Oligomerization of the mitochondrial protein voltage-dependent anion channel is coupled to the induction of apoptosis
- PMID: 20937774
- PMCID: PMC3004265
- DOI: 10.1128/MCB.00165-10
Oligomerization of the mitochondrial protein voltage-dependent anion channel is coupled to the induction of apoptosis
Abstract
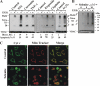
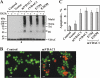
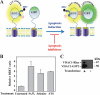
Accumulating evidence implicates that the voltage-dependent anion channel (VDAC) functions in mitochondrion-mediated apoptosis and as a critical player in the release of apoptogenic proteins, such as cytochrome c, triggering caspase activation and apoptosis. The mechanisms regulating cytochrome c release and the molecular architecture of the cytochrome c-conducting channel remain unknown. Here the relationship between VDAC oligomerization and the induction of apoptosis was examined. We demonstrated that apoptosis induction by various stimuli was accompanied by highly increased VDAC oligomerization, as revealed by cross-linking and directly monitored in living cells using bioluminescence resonance energy transfer technology. VDAC oligomerization was induced in all cell types and with all apoptosis inducers used, including staurosporine, curcumin, As(2)O(3), etoposide, cisplatin, selenite, tumor necrosis factor alpha (TNF-α), H(2)O(2), and UV irradiation, all acting through different mechanisms yet all involving mitochondria. Moreover, correlation between the levels of VDAC oligomerization and apoptosis was observed. Furthermore, the apoptosis inhibitor 4,4'-diisothiocyanostilbene-2,2'-disulfonic acid (DIDS) inhibited VDAC oligomerization. Finally, a caspase inhibitor had no effect on VDAC oligomerization and cytochrome c release. We propose that VDAC oligomerization is involved in mitochondrion-mediated apoptosis and may represent a general mechanism common to numerous apoptogens acting via different initiating cascades. Thus, targeting the oligomeric status of VDAC, and hence apoptosis, offers a therapeutic strategy for combating cancers and neurodegenerative diseases.
Figures

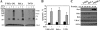



References
-
- Abu-Hamad, S., N. Arbel, D. Calo, L. Arzoine, A. Israelson, N. Keinan, R. Ben-Romano, O. Friedman, and V. Shoshan-Barmatz. 2009. The VDAC1 N-terminus is essential both for apoptosis and the protective effect of anti-apoptotic proteins. J. Cell Sci. 122:1906-1916. - PubMed
-
- Abu-Hamad, S., H. Zaid, A. Israelson, E. Nahon, and V. Shoshan-Barmatz. 2008. Hexokinase-I protection against apoptotic cell death is mediated via interaction with the voltage-dependent anion channel-1: mapping the site of binding. J. Biol. Chem. 19:13482-13490. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
