An oracle: antituberculosis pharmacokinetics-pharmacodynamics, clinical correlation, and clinical trial simulations to predict the future
- PMID: 20937778
- PMCID: PMC3019641
- DOI: 10.1128/AAC.00749-10
An oracle: antituberculosis pharmacokinetics-pharmacodynamics, clinical correlation, and clinical trial simulations to predict the future
Abstract
Antimicrobial pharmacokinetic-pharmacodynamic (PK/PD) science and clinical trial simulations have not been adequately applied to the design of doses and dose schedules of antituberculosis regimens because many researchers are skeptical about their clinical applicability. We compared findings of preclinical PK/PD studies of current first-line antituberculosis drugs to findings from several clinical publications that included microbiologic outcome and pharmacokinetic data or had a dose-scheduling design. Without exception, the antimicrobial PK/PD parameters linked to optimal effect were similar in preclinical models and in tuberculosis patients. Thus, exposure-effect relationships derived in the preclinical models can be used in the design of optimal antituberculosis doses, by incorporating population pharmacokinetics of the drugs and MIC distributions in Monte Carlo simulations. When this has been performed, doses and dose schedules of rifampin, isoniazid, pyrazinamide, and moxifloxacin with the potential to shorten antituberculosis therapy have been identified. In addition, different susceptibility breakpoints than those in current use have been identified. These steps outline a more rational approach than that of current methods for designing regimens and predicting outcome so that both new and older antituberculosis agents can shorten therapy duration.
Figures
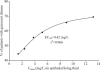

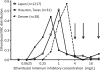
Comment in
-
Wild-type MIC distributions must be considered to set clinically meaningful susceptibility testing breakpoints for all bacterial pathogens, including Mycobacterium tuberculosis.Antimicrob Agents Chemother. 2011 Sep;55(9):4492-3; author reply 4493. doi: 10.1128/AAC.00232-11. Antimicrob Agents Chemother. 2011. PMID: 21849570 Free PMC article. No abstract available.
References
-
- Abdool Karim, S. S., K. Naidoo, A. Grobler, N. Padayatchi, C. Baxter, A. Gray, T. Gengiah, G. Nair, S. Bamber, A. Singh, M. Khan, J. Pienaar, W. El-Sadr, G. Friedland, and K. Q. Abdool. 2010. Timing of initiation of antiretroviral drugs during tuberculosis therapy. N. Engl. J. Med. 362:697-706. - PMC - PubMed
-
- Adams, C. H., C. J. Werely, T. C. Victor, E. G. Hoal, G. Rossouw, and P. D. van Helden. 2003. Allele frequencies for glutathione S-transferase and N-acetyltransferase 2 differ in African population groups and may be associated with oesophageal cancer or tuberculosis incidence. Clin. Chem. Lab. Med. 41:600-605. - PubMed
-
- Ambrose, P. G. 2006. Monte Carlo simulation in the evaluation of susceptibility breakpoints: predicting the future: insights from the Society of Infectious Diseases Pharmacists. Pharmacotherapy. 26:129-134. - PubMed
-
- Ambrose, P. G., S. M. Bhavnani, C. M. Rubino, A. Louie, T. Gumbo, A. Forrest, and G. L. Drusano. 2007. Pharmacokinetics-pharmacodynamics of antimicrobial therapy: it's not just for mice anymore. Clin. Infect. Dis. 44:79-86. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

