Novel compounds containing multiple guanide groups that bind the HIV coreceptor CXCR4
- PMID: 20937786
- PMCID: PMC3019677
- DOI: 10.1128/AAC.00709-10
Novel compounds containing multiple guanide groups that bind the HIV coreceptor CXCR4
Abstract
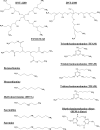
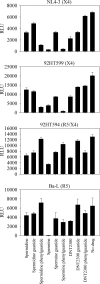
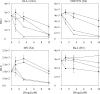
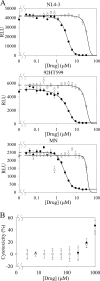
The G-protein-coupled receptor CXCR4 acts as a coreceptor for human immunodeficiency virus type 1 (HIV-1) infection, as well as being involved in signaling cell migration and proliferation. Compounds that block CXCR4 interactions have potential uses as HIV entry inhibitors to complement drugs such as maraviroc that block the alternate coreceptor CCR5 or in cancer therapy. The peptide T140, which contains five arginine residues, is the most potent antagonist of CXCR4 developed to date. In a search for nonpeptide CXCR4 ligands that could inhibit HIV entry, three series of compounds were synthesized from 12 linear and branched polyamines with 2, 3, 4, 6, or 8 amino groups, which were substituted to produce the corresponding guanidines, biguanides, or phenylguanides. The resulting compounds were tested for their ability to compete with T140 for binding to the human CXCR4 receptor expressed on mammalian cells. The most effective compounds bound CXCR4 with a 50% inhibitory concentration of 200 nM, and all of the compounds had very low cytotoxicity. Two series of compounds were then tested for their ability to inhibit the infection of TZM-bl cells with X4 and R5 strains of HIV-1. Spermine phenylguanide and spermidine phenylguanide inhibited infection by X4 strains, but not by R5 strains, at low micromolar concentrations. These results support further investigation and development of these compounds as HIV entry inhibitors.
Figures
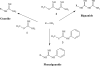


References
-
- Babcock, G. J., T. Mirzabekov, W. Wojtowicz, and J. Sodroski. 2001. Ligand binding characteristics of CXCR4 incorporated into paramagnetic proteoliposomes. J. Biol. Chem. 276:38433-38440. - PubMed
-
- Billick, E., C. Seibert, P. Pugach, T. Ketas, A. Trkola, M. J. Endres, N. J. Murgolo, E. Coates, G. R. Reyes, B. M. Baroudy, T. P. Sakmar, J. P. Moore, and S. E. Kuhmann. 2004. The differential sensitivity of human and rhesus macaque CCR5 to small-molecule inhibitors of human immunodeficiency virus type 1 entry is explained by a single amino acid difference and suggests a mechanism of action for these inhibitors. J. Virol. 78:4134-4144. - PMC - PubMed
-
- Catalone, B. J., T. M. Kish-Catalone, L. R. Budgeon, E. B. Neely, M. Ferguson, F. C. Krebs, M. K. Howett, M. Labib, R. Rando, and B. Wigdahl. 2004. Mouse model of cervicovaginal toxicity and inflammation for preclinical evaluation of topical vaginal microbicides. Antimicrob. Agents Chemother. 48:1837-1847. - PMC - PubMed
-
- De Clercq, E. 2009. The AMD3100 story: the path to the discovery of a stem cell mobilizer (Mozobil). Biochem. Pharmacol. 77:1655-1664. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

