Ca2+ release to lumen from ADP-sensitive phosphoenzyme E1PCa2 without bound K+ of sarcoplasmic reticulum Ca2+-ATPase
- PMID: 20937807
- PMCID: PMC2992300
- DOI: 10.1074/jbc.M110.183343
Ca2+ release to lumen from ADP-sensitive phosphoenzyme E1PCa2 without bound K+ of sarcoplasmic reticulum Ca2+-ATPase
Abstract
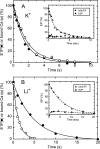
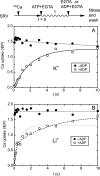
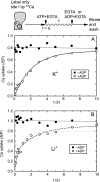
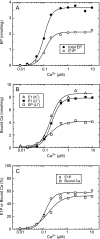
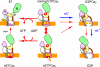
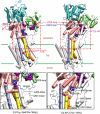
During Ca(2+) transport by sarcoplasmic reticulum Ca(2+)-ATPase, the conformation change of ADP-sensitive phosphoenzyme (E1PCa(2)) to ADP-insensitive phosphoenzyme (E2PCa(2)) is followed by rapid Ca(2+) release into the lumen. Here, we find that in the absence of K(+), Ca(2+) release occurs considerably faster than E1PCa(2) to E2PCa(2) conformation change. Therefore, the lumenal Ca(2+) release pathway is open to some extent in the K(+)-free E1PCa(2) structure. The Ca(2+) affinity of this E1P is as high as that of the unphosphorylated ATPase (E1), indicating the Ca(2+) binding sites are not disrupted. Thus, bound K(+) stabilizes the E1PCa(2) structure with occluded Ca(2+), keeping the Ca(2+) pathway to the lumen closed. We found previously (Yamasaki, K., Wang, G., Daiho, T., Danko, S., and Suzuki, H. (2008) J. Biol. Chem. 283, 29144-29155) that the K(+) bound in E2P reduces the Ca(2+) affinity essential for achieving the high physiological Ca(2+) gradient and to fully open the lumenal Ca(2+) gate for rapid Ca(2+) release (E2PCa(2) → E2P + 2Ca(2+)). These findings show that bound K(+) is critical for stabilizing both E1PCa(2) and E2P structures, thereby contributing to the structural changes that efficiently couple phosphoenzyme processing and Ca(2+) handling.
Figures

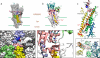
Similar articles
-
Roles of Tyr122-hydrophobic cluster and K+ binding in Ca2+ -releasing process of ADP-insensitive phosphoenzyme of sarcoplasmic reticulum Ca2+ -ATPase.J Biol Chem. 2008 Oct 24;283(43):29144-55. doi: 10.1074/jbc.M804596200. Epub 2008 Aug 26. J Biol Chem. 2008. PMID: 18728008 Free PMC article.
-
Critical role of Glu40-Ser48 loop linking actuator domain and first transmembrane helix of Ca2+-ATPase in Ca2+ deocclusion and release from ADP-insensitive phosphoenzyme.J Biol Chem. 2007 Nov 23;282(47):34429-47. doi: 10.1074/jbc.M707665200. Epub 2007 Sep 19. J Biol Chem. 2007. PMID: 17881350
-
Stable structural analog of Ca2+-ATPase ADP-insensitive phosphoenzyme with occluded Ca2+ formed by elongation of A-domain/M1'-linker and beryllium fluoride binding.J Biol Chem. 2010 Aug 6;285(32):24538-47. doi: 10.1074/jbc.M110.144535. Epub 2010 Jun 7. J Biol Chem. 2010. PMID: 20529842 Free PMC article.
-
Distinct natures of beryllium fluoride-bound, aluminum fluoride-bound, and magnesium fluoride-bound stable analogues of an ADP-insensitive phosphoenzyme intermediate of sarcoplasmic reticulum Ca2+-ATPase: changes in catalytic and transport sites during phosphoenzyme hydrolysis.J Biol Chem. 2004 Apr 9;279(15):14991-8. doi: 10.1074/jbc.M313363200. Epub 2004 Jan 30. J Biol Chem. 2004. PMID: 14754887
-
Formation of the stable structural analog of ADP-sensitive phosphoenzyme of Ca2+-ATPase with occluded Ca2+ by beryllium fluoride: structural changes during phosphorylation and isomerization.J Biol Chem. 2009 Aug 21;284(34):22722-35. doi: 10.1074/jbc.M109.029702. Epub 2009 Jun 26. J Biol Chem. 2009. PMID: 19561071 Free PMC article.
Cited by
-
Assembly of a Tyr122 Hydrophobic Cluster in Sarcoplasmic Reticulum Ca2+-ATPase Synchronizes Ca2+ Affinity Reduction and Release with Phosphoenzyme Isomerization.J Biol Chem. 2015 Nov 13;290(46):27868-79. doi: 10.1074/jbc.M115.693770. Epub 2015 Oct 6. J Biol Chem. 2015. PMID: 26442589 Free PMC article.
-
Roles of long-range electrostatic domain interactions and K+ in phosphoenzyme transition of Ca2+-ATPase.J Biol Chem. 2013 Jul 12;288(28):20646-57. doi: 10.1074/jbc.M113.482711. Epub 2013 Jun 4. J Biol Chem. 2013. PMID: 23737524 Free PMC article.
References
-
- Hasselbach W., Makinose M. (1961) Biochem. Z. 333, 518–528 - PubMed
-
- Inesi G., Sumbilla C., Kirtley M. E. (1990) Physiol. Rev. 70, 749–760 - PubMed
-
- Møller J. V., Juul B., le Maire M. (1996) Biochim. Biophys. Acta 1286, 1–51 - PubMed
-
- MacLennan D. H., Rice W. J., Green N. M. (1997) J. Biol. Chem. 272, 28815–28818 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

