Angiotensin-converting enzyme overexpression in mouse myelomonocytic cells augments resistance to Listeria and methicillin-resistant Staphylococcus aureus
- PMID: 20937811
- PMCID: PMC2998083
- DOI: 10.1074/jbc.M110.163782
Angiotensin-converting enzyme overexpression in mouse myelomonocytic cells augments resistance to Listeria and methicillin-resistant Staphylococcus aureus
Abstract
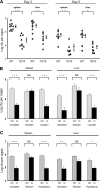
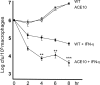
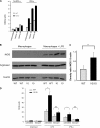
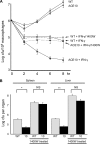
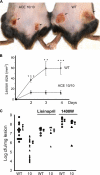
Gene targeting in ES cells was used to substitute control of angiotensin converting enzyme (ACE) expression from the endogenous promoter to the mouse c-fms promoter. The result is an animal model called ACE 10/10 in which ACE is overexpressed by monocytes, macrophages, and other myelomonocytic lineage cells. To study the immune response of these mice to bacterial infection, we challenged them with Listeria monocytogenes or methicillin-resistant Staphylococcus aureus (MRSA). ACE 10/10 mice have a significantly enhanced immune response to both bacteria in vivo and in vitro. For example, 5 days after Listeria infection, the spleen and liver of ACE 10/10 mice had 8.0- and 5.2-fold less bacteria than wild type mice (WT). In a model of MRSA skin infection, ACE 10/10 mice had 50-fold less bacteria than WT mice. Histologic examination showed a prominent infiltrate of ACE-positive mononuclear cells in the skin lesions from ACE 10/10. Increased bacterial resistance in ACE 10/10 is directly due to overexpression of ACE, as it is eliminated by an ACE inhibitor. Critical to increased immunity in ACE 10/10 is the overexpression of iNOS and reactive nitrogen intermediates, as inhibition of iNOS by the inhibitor 1400W eliminated all in vitro and in vivo differences in innate bacterial resistance between ACE 10/10 and WT mice. Increased resistance to MRSA was transferable by bone marrow transplantation. The overexpression of ACE and iNOS by myelomonocytic cells substantially boosts innate immunity and may represent a new means to address serious bacterial infections.
Figures


References
-
- Corvol P., Williams T. A., Soubrier F. (1995) Methods Enzymol. 248, 283–305 - PubMed
-
- Bernstein K. E., Xiao H. D., Frenzel K., Li P., Shen X. Z., Adams J. W., Fuchs S. (2005) Circ. Res. 96, 1135–1144 - PubMed
-
- Leehey D. J., Singh A. K., Alavi N., Singh R. (2000) Kidney Int. Suppl. 77, S93–S98 - PubMed
-
- Metzger R., Bohle R. M., Chumachenko P., Danilov S. M., Franke F. E. (2000) Atherosclerosis 150, 21–31 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Miscellaneous

