Long range interactions regulate Igf2 gene transcription during skeletal muscle differentiation
- PMID: 20937833
- PMCID: PMC2998108
- DOI: 10.1074/jbc.M110.160986
Long range interactions regulate Igf2 gene transcription during skeletal muscle differentiation
Abstract
The differentiation, maintenance, and repair of skeletal muscle is controlled by interactions between genetically determined transcriptional programs regulated by myogenic transcription factors and environmental cues activated by growth factors and hormones. Signaling through the insulin-like growth factor 1 (IGF1) receptor by locally produced IGF2 defines one such pathway that is critical for normal muscle growth and for regeneration after injury. IGF2 gene and protein expression are induced as early events in muscle differentiation, but the responsible molecular mechanisms are unknown. Here we characterize a distal DNA element within the imprinted mouse Igf2-H19 locus with properties of a muscle transcriptional enhancer. We find that this region undergoes a transition to open chromatin during differentiation, whereas adjacent chromatin remains closed, and that it interacts in differentiating muscle nuclei but not in mesenchymal precursor cells with the Igf2 gene found more than 100 kb away, suggesting that chromatin looping or sliding to bring the enhancer in proximity to Igf2 promoters is also an early event in muscle differentiation. Because this element directly stimulates the transcriptional activity of an Igf2 promoter-reporter gene in differentiating myoblasts, our results indicate that we have identified a bona fide distal transcriptional enhancer that supports Igf2 gene activation in skeletal muscle cells. Because this DNA element is conserved in the human IGF2-H19 locus, our results further suggest that its muscle enhancer function also is conserved among different mammalian species.
Figures



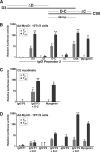



References
-
- Lassar A., Münsterberg A. (1994) Curr. Opin. Cell Biol. 6, 432–442 - PubMed
-
- Naya F. J., Olson E. (1999) Curr. Opin Cell Biol. 11, 683–688 - PubMed
-
- McKinsey T. A., Zhang C. L., Olson E. N. (2002) Trends Biochem. Sci 27, 40–47 - PubMed
-
- Palacios D., Puri P. L. (2006) J. Cell. Physiol. 207, 1–11 - PubMed
-
- Glass D. J. (2003) Nat. Cell Biol. 5, 87–90 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

