Peptidylarginine deiminase 2 suppresses inhibitory {kappa}B kinase activity in lipopolysaccharide-stimulated RAW 264.7 macrophages
- PMID: 20937835
- PMCID: PMC3000946
- DOI: 10.1074/jbc.M110.170290
Peptidylarginine deiminase 2 suppresses inhibitory {kappa}B kinase activity in lipopolysaccharide-stimulated RAW 264.7 macrophages
Abstract
Peptidylarginine deiminases (PADs) are enzymes that convert arginine to citrulline in proteins. In this study, we examined PAD-mediated citrullination and its effect on pro-inflammatory activity in the macrophage cell line RAW 264.7. Citrullination of 45-65-kDa proteins was induced when cells were treated with lipopolysaccharide (LPS; 1 μg/ml). Protein citrullination was suppressed by the intracellular calcium chelator BAPTA/AM (30 μM). LPS treatment up-regulated COX-2 levels in cells. Interestingly, overexpressing PAD2 reduced LPS-mediated COX-2 up-regulation by 50%. PAD2 overexpression also reduced NF-κB activity, determined by NF-κB-driven luciferase activity. The effect of PAD2 on NF-κB activity was further examined by using HEK 293 cells transfected with NF-κB luciferase, IκB β/γ kinase (IKKβ/γ) subunits, and PAD2. IKKβ increased NF-κB activity, but this increase was markedly suppressed when PAD2 was present in cells. IKKβ-mediated NF-κB activation was further enhanced by IKKγ in the presence of calcium ionophore A23187. However, this stimulatory effect of IKKβ/γ was abolished by PAD2. Coimmunoprecipitation of cell lysates showed that IKKγ and PAD2 can coimmunoprecipitate in the presence of the Ca(2+) ionophore. IKKγ coimmunoprecipitated truncation mutants, PAD2(1-385) and PAD2(355-672). The substitution of Gln-358 (a putative ligand for Ca(2+) binding) with an Ala abolished coimmunoprecipitation. Conversely, PAD2 coimmunoprecipitated truncation mutants IKKγ(1-196) and IKKγ(197-419). In other experiments, treating RAW 264.7 cells with LPS induced citrullination in the immunoprecipitates of IKKγ. In vitro citrullination assay showed that incubation of purified PAD2 and IKKγ proteins in the presence of Ca(2+) citrullinated IKKγ. These results demonstrate that PAD2 interacts with IKKγ and suppresses NF-κB activity.
Figures
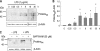

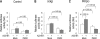


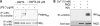
References
-
- Rogers G. E. (1962) Nature 194, 1149–1151 - PubMed
-
- Vossenaar E. R., Zendman A. J., van Venrooij W. J., Pruijn G. J. (2003) Bioessays 25, 1106–1118 - PubMed
-
- Tarcsa E., Marekov L. N., Mei G., Melino G., Lee S. C., Steinert P. M. (1996) J. Biol. Chem. 271, 30709–30716 - PubMed
-
- Arita K., Hashimoto H., Shimizu T., Nakashima K., Yamada M., Sato M. (2004) Nat. Struct. Mol. Biol. 11, 777–783 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

