CCR10 is important for the development of skin-specific gammadeltaT cells by regulating their migration and location
- PMID: 20937851
- PMCID: PMC3037513
- DOI: 10.4049/jimmunol.1001612
CCR10 is important for the development of skin-specific gammadeltaT cells by regulating their migration and location
Abstract
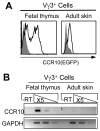
Unlike conventional αβ T cells, which preferentially reside in secondary lymphoid organs for adaptive immune responses, various subsets of unconventional T cells, such as the γδ T cells with innate properties, preferentially reside in epithelial tissues as the first line of defense. However, mechanisms underlying their tissue-specific development are not well understood. We report in this paper that among different thymic T cell subsets fetal thymic precursors of the prototypic skin intraepithelial Vγ3(+) T lymphocytes (sIELs) were selected to display a unique pattern of homing molecules, including a high level of CCR10 expression that was important for their development into sIELs. In fetal CCR10-knockout mice, the Vγ3(+) sIEL precursors developed normally in the thymus but were defective in migrating into the skin. Although the earlier defect in skin-seeding by sIEL precursors was partially compensated for by their normal expansion in the skin of adult CCR10-knockout mice, the Vγ3(+) sIELs displayed abnormal morphology and increasingly accumulated in the dermal region of the skin. These findings provide definite evidence that CCR10 is important in sIEL development by regulating the migration of sIEL precursors and their maintenance in proper regions of the skin and support the notion that unique homing properties of different thymic T cell subsets play an important role in their peripheral location.
Figures


References
-
- Hayday AC. [gamma][delta] cells: a right time and a right place for a conserved third way of protection. Annual Review of Immunology. 2000;18:975–1026. - PubMed
-
- Raulet D, Spencer D, Hsiang Y-H, Goldman J, Bix M, Liao N-S, Zijlstra M, Jaenisch R, Correa I. Control of γδ T cell development. Immunol. Rev. 1991;120:185–204. - PubMed
-
- Xiong N, Raulet DH. Development and selection of gammadelta T cells. Immunol Rev. 2007;215:15–31. - PubMed
-
- Girardi M, Oppenheim DE, Steele CR, Lewis JM, Glusac E, Filler R, Hobby P, Sutton B, Tigelaar RE, Hayday AC. Regulation of cutaneous malignancy by gammadelta T cells. Science. 2001;294:605–609. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

