Glycosylation of surface Ig creates a functional bridge between human follicular lymphoma and microenvironmental lectins
- PMID: 20937880
- PMCID: PMC2972945
- DOI: 10.1073/pnas.1009388107
Glycosylation of surface Ig creates a functional bridge between human follicular lymphoma and microenvironmental lectins
Abstract
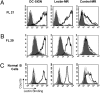
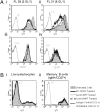
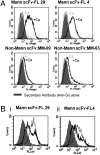
Surface Ig (sIg) of follicular lymphoma (FL) is vital for tumor cell survival. We found previously that the Ig in FL is unusual, because the variable region genes carry sequence motifs for N-glycan addition. These are introduced by somatic mutation and are tumor specific. Unexpectedly, added glycans terminate at high mannose, suggesting a potentially important interaction of FL cells with mannose-binding lectins of the innate immune system. We have now identified mannosylated IgM at the surface of primary lymphoma cells. Recombinant lectin domains of the mannose receptor (MR) or DC-SIGN bind mannosylated Igs in vitro and bind to FL cells, signaling sIgM-associated increases in intracellular Ca(2+). Lectins also bind to normal B cells but fail to signal. In contrast, anti-Ig signaled similarly in both FL and normal B cells. Mannosylation patterns were mimicked by FL Ig-derived single-chain Fvs (scFv), providing probes for potential receptors. Mannosylated scFv bound specifically to the lectin domains of the MR and DC-SIGN and blocked signaling. Mannosylated scFv also bound to DC-SIGN on the surface of dendritic cells. This unique lymphoma-specific interaction of sIg with lectins of innate immunity reveals a potential route for microenvironmental support of tumor cells, mediated via the key B-cell receptor.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
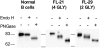

References
-
- Tsujimoto Y, Cossman J, Jaffe E, Croce CM. Involvement of the bcl-2 gene in human follicular lymphoma. Science. 1985;228:1440–1443. - PubMed
-
- Limpens J, et al. Lymphoma-associated translocation t(14;18) in blood B cells of normal individuals. Blood. 1995;85:2528–2536. - PubMed
-
- Zhu D, et al. Acquisition of potential N-glycosylation sites in the immunoglobulin variable region by somatic mutation is a distinctive feature of follicular lymphoma. Blood. 2002;99:2562–2568. - PubMed
-
- Zhu D, Ottensmeier CH, Du MQ, McCarthy H, Stevenson FK. Incidence of potential glycosylation sites in immunoglobulin variable regions distinguishes between subsets of Burkitt's lymphoma and mucosa-associated lymphoid tissue lymphoma. Br J Haematol. 2003;120:217–222. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

