Contribution of calcium-dependent facilitation to synaptic plasticity revealed by migraine mutations in the P/Q-type calcium channel
- PMID: 20937883
- PMCID: PMC2972937
- DOI: 10.1073/pnas.1009500107
Contribution of calcium-dependent facilitation to synaptic plasticity revealed by migraine mutations in the P/Q-type calcium channel
Abstract
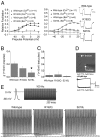
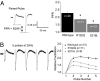
The dynamics, computational power, and strength of neural circuits are essential for encoding and processing information in the CNS and rely on short and long forms of synaptic plasticity. In a model system, residual calcium (Ca(2+)) in presynaptic terminals can act through neuronal Ca(2+) sensor proteins to cause Ca(2+)-dependent facilitation (CDF) of P/Q-type channels and induce short-term synaptic facilitation. However, whether this is a general mechanism of plasticity at intact central synapses and whether mutations associated with human disease affect this process have not been described to our knowledge. In this report, we find that, in both exogenous and native preparations, gain-of-function missense mutations underlying Familial Hemiplegic Migraine type 1 (FHM-1) occlude CDF of P/Q-type Ca(2+) channels. In FHM-1 mutant mice, the alteration of P/Q-type channel CDF correlates with reduced short-term synaptic facilitation at cerebellar parallel fiber-to-Purkinje cell synapses. Two-photon imaging suggests that P/Q-type channels at parallel fiber terminals in FHM-1 mice are in a basally facilitated state. Overall, the results provide evidence that FHM-1 mutations directly affect both P/Q-type channel CDF and synaptic plasticity and that together likely contribute toward the pathophysiology underlying FHM-1. The findings also suggest that P/Q-type channel CDF is an important mechanism required for normal synaptic plasticity at a fast synapse in the mammalian CNS.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


References
-
- Zucker RS, Regehr WG. Short-term synaptic plasticity. Annu Rev Physiol. 2002;64:355–405. - PubMed
-
- Xu J, Wu LG. The decrease in the presynaptic calcium current is a major cause of short-term depression at a calyx-type synapse. Neuron. 2005;46:633–645. - PubMed
-
- Tsujimoto T, Jeromin A, Saitoh N, Roder JC, Takahashi T. Neuronal calcium sensor 1 and activity-dependent facilitation of P/Q-type calcium currents at presynaptic nerve terminals. Science. 2002;295:2276–2279. - PubMed
-
- Mochida S, Few AP, Scheuer T, Catterall WA. Regulation of presynaptic Ca(V)2.1 channels by Ca2+ sensor proteins mediates short-term synaptic plasticity. Neuron. 2008;57:210–216. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

