Structure and mechanism of the complex between cytochrome P4503A4 and ritonavir
- PMID: 20937904
- PMCID: PMC2973003
- DOI: 10.1073/pnas.1010693107
Structure and mechanism of the complex between cytochrome P4503A4 and ritonavir
Abstract
Ritonavir is a HIV protease inhibitor routinely prescribed to HIV patients that also potently inactivates cytochrome P4503A4 (CYP3A4), the major human drug-metabolizing enzyme. By inhibiting CYP3A4, ritonavir increases plasma concentrations of other anti-HIV drugs oxidized by CYP3A4 thereby improving clinical efficacy. Despite the importance and wide use of ritonavir in anti-HIV therapy, the precise mechanism of CYP3A4 inhibition remains unclear. The available data are inconsistent and suggest that ritonavir acts as a mechanism-based, competitive or mixed competitive-noncompetitive CYP3A4 inactivator. To resolve this controversy and gain functional and structural insights into the mechanism of CYP3A4 inhibition, we investigated the ritonavir binding reaction by kinetic and equilibrium analysis, elucidated how the drug affects redox properties of the hemoprotein, and determined the 2.0 Å X-ray structure of the CYP3A4-ritonavir complex. Our results show that ritonavir is a type II ligand that perfectly fits into the CYP3A4 active site cavity and irreversibly binds to the heme iron via the thiazole nitrogen, which decreases the redox potential of the protein and precludes its reduction with the redox partner, cytochrome P450 reductase.
Conflict of interest statement
The authors declare no conflict of interest.
Figures




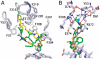
References
-
- Ortiz de Montellano P, editor. Cytochrome P450: structure, mechanism, and biochemistry. New York: Kluwer Academic/Plenum Publishers; 2005.
-
- Williams PA, et al. Crystal structures of human cytochrome P450 3A4 bound to metyrapone and progesterone. Science. 2004;305:683–686. - PubMed
-
- Guengerich FP, et al. Twenty years of biochemistry of human P450s: purification, expression, mechanism, and relevance to drugs. Drug Metab Dispos. 1998;26:1175–1178. - PubMed
-
- Xu L, Desai MC. Pharmacokinetic enhancers for HIV drugs. Curr Opin Investig D. 2009;10:775–786. - PubMed
-
- Kumar GN, Rodrigues AD, Buko AM, Denissen JF. Cytochrome P450-mediated metabolism of the HIV-1 protease inhibitor ritonavir (ABT-538) in human liver microsomes. J Pharmacol Exp Ther. 1996;277:423–431. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

