Alternative splicing of SLC39A14 in colorectal cancer is regulated by the Wnt pathway
- PMID: 20938052
- PMCID: PMC3013455
- DOI: 10.1074/mcp.M110.002998
Alternative splicing of SLC39A14 in colorectal cancer is regulated by the Wnt pathway
Abstract
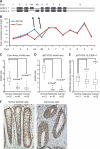
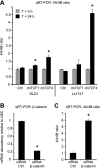
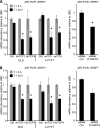
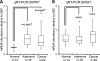
Alternative splicing is a crucial step in the generation of protein diversity and its misregulation is observed in many human cancer types. By analyzing 143 colorectal samples using exon arrays, SLC39A14, a divalent cation transporter, was identified as being aberrantly spliced in tumor samples. SLC39A14 contains two mutually exclusive exons 4A and 4B and the exon 4A/4B ratio was significantly altered in adenomas (p = 3.6 × 10(-10)) and cancers (p = 9.4 × 10(-11)), independent of microsatellite stability status. The findings were validated in independent exon array data sets and by quantitative real-time reverse-transcription PCR (qRT-PCR). Aberrant Wnt signaling is a hallmark of colorectal tumorigenesis and is characterized by nuclear β-catenin. Experimental inactivation of Wnt signaling in DLD1 and Ls174T cells by knockdown of β-catenin or overexpression of dominant negative TCFs (TCF1 and TCF4) altered the 4A/4B ratio, indicating that SLC39A14 splicing is regulated by the Wnt pathway. An altered 4A/4B ratio was also observed in gastric and lung cancer where Wnt signaling is also known to be aberrantly activated. The splicing factor SRSF1 and its regulator, the kinase SRPK1, were found to be deregulated upon Wnt inactivation in colorectal carcinoma cells. SRPK1 was also found up-regulated in both adenoma samples (p = 1.5 × 10(-5)) and cancer samples (p = 5 × 10(-4)). In silico splicing factor binding analysis predicted SRSF1 to bind predominantly to the cancer associated exon 4B, hence, it was hypothesized that SRPK1 activates SRSF1 through phosphorylation, followed by SRSF1 binding to exon 4B and regulation of SLC39A14 splicing. Indeed, siRNA-mediated knockdown of SRPK1 and SRSF1 in DLD1 and SW480 colorectal cancer cells led to a change in the 4A/4B isoform ratio, supporting a role of these factors in the regulation of SLC39A14 splicing. In conclusion, alternative splicing of SLC39A14 was identified in colorectal tumors and found to be regulated by the Wnt pathway, most likely through regulation of SRPK1 and SRSF1.
Figures

References
-
- Bienz M., Clevers H. (2000) Linking colorectal cancer to Wnt signaling. Cell 103, 311–320 - PubMed
-
- van de Wetering M., Sancho E., Verweij C., de Lau W., Oving I., Hurlstone A., van der Horn K., Batlle E., Coudreuse D., Haramis A. P., Tjon-Pon-Fong M., Moerer P., van den Born M., Soete G., Pals S., Eilers M., Medema R., Clevers H. (2002) The beta-catenin/TCF-4 complex imposes a crypt progenitor phenotype on colorectal cancer cells. Cell 111, 241–250 - PubMed
-
- Van Scoyk M., Randall J., Sergew A., Williams L. M., Tennis M., Winn R. A. (2008) Wnt signaling pathway and lung disease. Transl Res. 151, 175–180 - PubMed
-
- Clements W. M., Wang J., Sarnaik A., Kim O. J., MacDonald J., Fenoglio-Preiser C., Groden J., Lowy A. M. (2002) beta-Catenin mutation is a frequent cause of Wnt pathway activation in gastric cancer. Cancer Res. 62, 3503–3506 - PubMed
-
- Yue W., Sun Q., Dacic S., Landreneau R. J., Siegfried J. M., Yu J., Zhang L. (2008) Downregulation of Dkk3 activates beta-catenin/TCF-4 signaling in lung cancer. Carcinogenesis 29, 84–92 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

