Mechanisms of disease: Helicobacter pylori virulence factors
- PMID: 20938460
- PMCID: PMC3137895
- DOI: 10.1038/nrgastro.2010.154
Mechanisms of disease: Helicobacter pylori virulence factors
Abstract
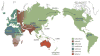
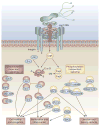
Helicobacter pylori plays an essential role in the development of various gastroduodenal diseases; however, only a small proportion of people infected with H. pylori develop these diseases. Some populations that have a high prevalence of H. pylori infection also have a high incidence of gastric cancer (for example, in East Asia), whereas others do not (for example, in Africa and South Asia). Even within East Asia, the incidence of gastric cancer varies (decreasing in the south). H. pylori is a highly heterogeneous bacterium and its virulence varies geographically. Geographic differences in the incidence of gastric cancer can be explained, at least in part, by the presence of different types of H. pylori virulence factor, especially CagA, VacA and OipA. However, it is still unclear why the pathogenicity of H. pylori increased as it migrated from Africa to East Asia during the course of evolution. H. pylori infection is also thought to be involved in the development of duodenal ulcer, which is at the opposite end of the disease spectrum to gastric cancer. This discrepancy can be explained in part by the presence of H. pylori virulence factor DupA. Despite advances in our understanding of the development of H. pylori-related diseases, further work is required to clarify the roles of H. pylori virulence factors.
Conflict of interest statement
The author declares no competing interests.
Figures



References
-
- International Agency for Research on Cancer. The Globocan Project. 2010 [online], http://globocan.iarc.fr/
-
- Malaty HM. Epidemiology of Helicobacter pylori infection. Best Pract Res Clin Gastroenterol. 2007;21:205–214. - PubMed
-
- El-Omar EM. Role of host genes in sporadic gastric cancer. Best Pract Res Clin Gastroenterol. 2006;20:675–686. - PubMed
-
- Graham DY. Helicobacter pylori infection in the pathogenesis of duodenal ulcer and gastric cancer: a model. Gastroenterology. 1997;113:1983–1991. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

