When you can't trust the DNA: RNA editing changes transcript sequences
- PMID: 20938709
- PMCID: PMC11114842
- DOI: 10.1007/s00018-010-0538-9
When you can't trust the DNA: RNA editing changes transcript sequences
Abstract
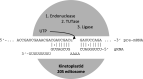
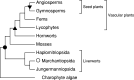
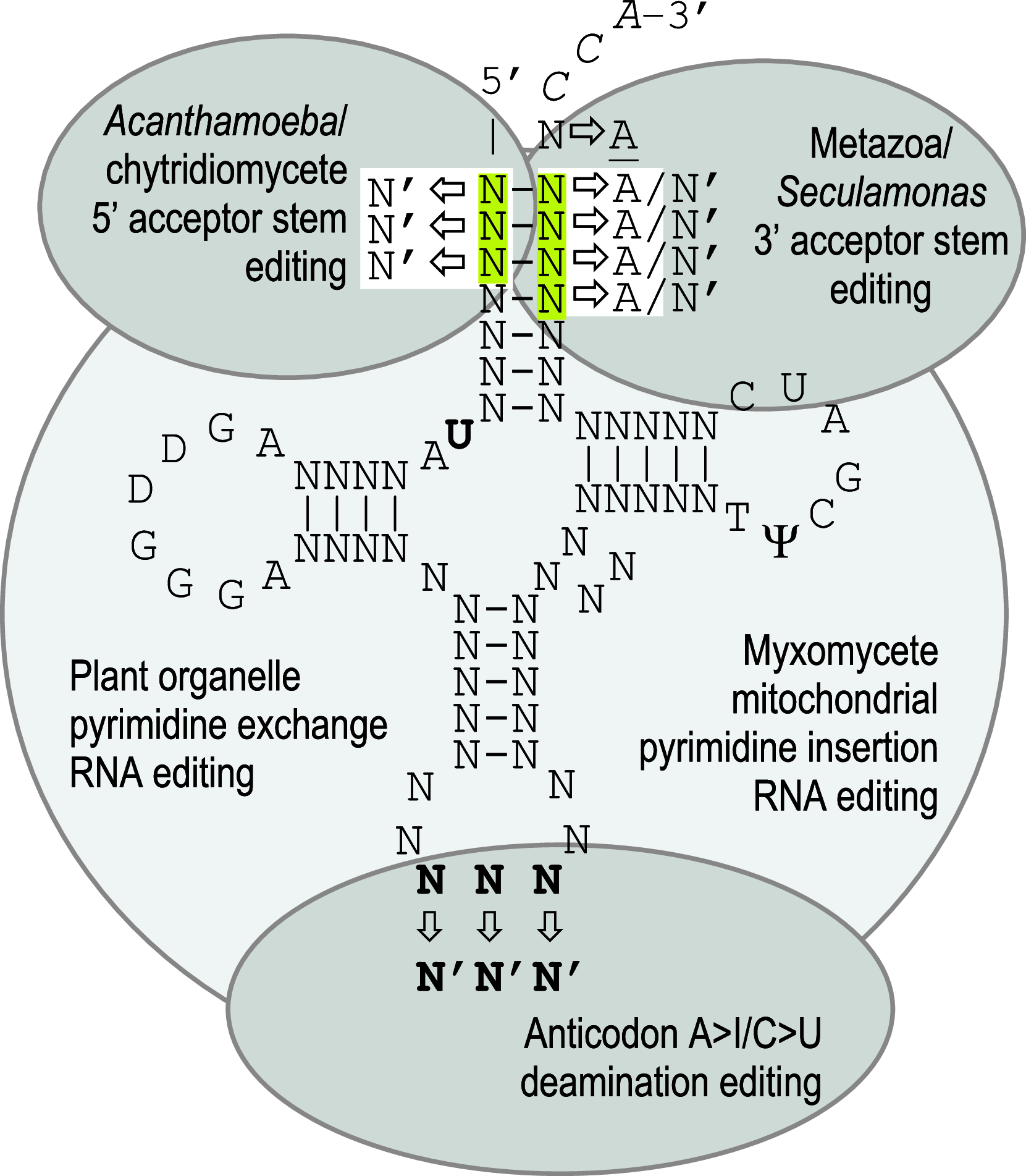
RNA editing describes targeted sequence alterations in RNAs so that the transcript sequences differ from their DNA template. Since the original discovery of RNA editing in trypanosomes nearly 25 years ago more than a dozen such processes of nucleotide insertions, deletions, and exchanges have been identified in evolutionarily widely separated groups of the living world including plants, animals, fungi, protists, bacteria, and viruses. In many cases gene expression in mitochondria is affected, but RNA editing also takes place in chloroplasts and in nucleocytosolic genetic environments. While some RNA editing systems largely seem to repair defect genes (cryptogenes), others have obvious functions in modulating gene activities. The present review aims for an overview on the current states of research in the different systems of RNA editing by following a historic timeline along the respective original discoveries.
Figures

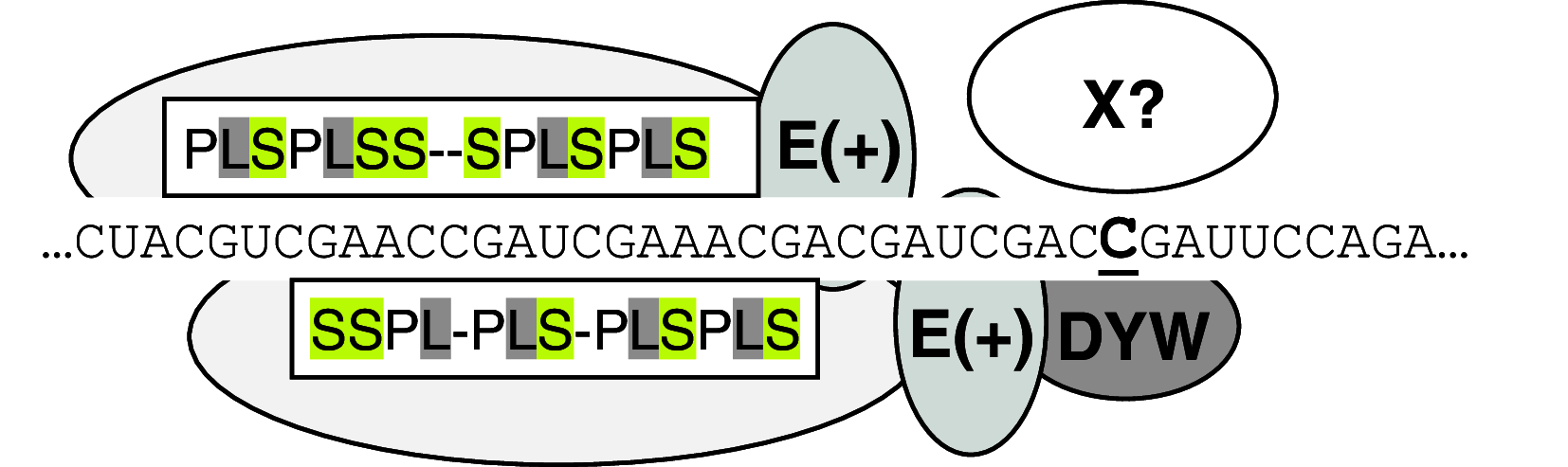
References
-
- Mandal D, Köhrer C, Su D, Russell SP, Krivos K, Castleberry CM, Blum P, Limbach PA, Söll D, RajBhandary UL. Agmatidine, a modified cytidine in the anticodon of archaeal tRNAIle, base pairs with adenosine but not with guanosine. Proc Natl Acad Sci USA. 2010;107:2872–2877. doi: 10.1073/pnas.0914869107. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

