Circulating high sensitivity troponin T in severe sepsis and septic shock: distribution, associated factors, and relation to outcome
- PMID: 20938765
- PMCID: PMC3020309
- DOI: 10.1007/s00134-010-2051-x
Circulating high sensitivity troponin T in severe sepsis and septic shock: distribution, associated factors, and relation to outcome
Abstract
Purpose: To assess the clinical utility of a recently developed highly sensitive cardiac troponin T (hs-cTnT) assay for providing prognostic information on patients with sepsis.
Methods: cTnT levels were measured by the novel hs-cTnT assay at two time points (inclusion and 72 h thereafter) in a subgroup of patients from the FINNSEPSIS study and associations with clinical outcomes were examined. Results for the hs-cTnT assay were compared to those of the established fourth-generation cTnT assay.
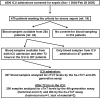
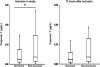
Results: cTnT measured by the fourth-generation and hs-cTnT assay was detectable in 124 (60%) and 207 (100%) patients, respectively, on inclusion in this study. hs-cTnT levels on inclusion correlated with several indices of risk in sepsis, including the simplified acute physiology score (SAPS) II and sequential organ failure assessment (SOFA) scores. The level of hs-cTnT on inclusion was higher in hospital non-survivors (n = 47) than survivors (n = 160) (median 0.054 [Q1-3, 0.022-0.227] versus 0.035 [0.015-0.111] μg/L, P = 0.047), but hs-cTnT level was not an independent predictor of in-hospital mortality. hs-cTnT levels on inclusion were also higher in patients with septic shock during the hospitalization (0.044 [0.024-0.171] versus 0.033 [0.012-0.103] μg/L, P = 0.03), while this was not the case for the fourth-generation cTnT assay or NT-proBNP levels.
Conclusions: Circulating hs-cTnT is present in patients with severe sepsis and septic shock, associates with disease severity and survival, but does not add to SAPS II score for prediction of mortality. hs-cTnT measurement could still have a role in sepsis as an early marker of shock.
Figures


References
-
- Fernandes CJ Jr, Akamine N, Knobel E (2008) Myocardial depression in sepsis. Shock 30 Suppl 1:S14–S17 - PubMed
-
- Morrow DA, Cannon CP, Jesse RL, Newby LK, Ravkilde J, Storrow AB, Wu AH, Christenson RH, National Academy of Clinical Biochemistry National Academy of Clinical Biochemistry laboratory medicine practice guidelines: clinical characteristics and utilization of biochemical markers in acute coronary syndromes. Circulation. 2007;115:e356–e375. doi: 10.1161/CIRCULATIONAHA.106.683110. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials

