An abrupt developmental shift in callosal modulation of sleep-related spindle bursts coincides with the emergence of excitatory-inhibitory balance and a reduction of somatosensory cortical plasticity
- PMID: 20939660
- PMCID: PMC2955326
- DOI: 10.1037/a0020774
An abrupt developmental shift in callosal modulation of sleep-related spindle bursts coincides with the emergence of excitatory-inhibitory balance and a reduction of somatosensory cortical plasticity
Abstract
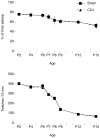
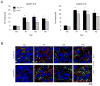
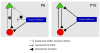
Transecting the corpus callosum of postnatal day (P)1-6 rats disinhibits the production of spindle bursts (SBs) within primary somatosensory cortex (S1), most notably during periods of sleep-related myoclonic twitching. Here we investigated developmental changes in this callosally mediated disinhibition and its association with cortical plasticity. Recordings in P2-15 subjects revealed that callosotomy-induced disinhibition is a transient feature of early development that disappears abruptly after P6. This abrupt switch was accompanied by sharp decreases in myoclonic twitching and equally sharp increases in spontaneous SBs and in the number of GABAergic and glutamatergic presynaptic terminals in S1. Expression of the K+Cl- cotransporter 2 (KCC2) also increased across these ages. To determine whether these developmental changes are associated with alterations in cortical plasticity, pups were callosotomized at P1, P6, or P8, and tested over the subsequent week. Regardless of age, callosotomy immediately disrupted SBs evoked by forepaw stimulation. Over the next week, the P1 and P6 callosotomy groups exhibited full recovery of function; in contrast, the P8 group did not exhibit recovery of function, thus indicating an abrupt decrease in cortical plasticity between P6 and P8. Together, our data demonstrate that callosotomy-induced disinhibition is a transient phenomenon whose disappearance coincides with the onset of increased intrinsic connectivity, establishment of excitatory-inhibitory balance, and diminished plasticity in S1. Accordingly, our findings indicate that callosotomy-induced disinhibition of twitch-related SBs is a bioassay of somatosensory cortical plasticity and, in addition, support the hypothesis that myoclonic twitches, like retinal waves, actively contribute to cortical development and plasticity.
(PsycINFO Database Record (c) 2010 APA, all rights reserved).
Figures

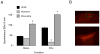



Similar articles
-
The corpus callosum modulates spindle-burst activity within homotopic regions of somatosensory cortex in newborn rats.Eur J Neurosci. 2008 Oct;28(8):1457-66. doi: 10.1111/j.1460-9568.2008.06461.x. Eur J Neurosci. 2008. PMID: 18973571 Free PMC article.
-
Removal of GABAergic inhibition alters subthreshold input in neurons in forepaw barrel subfield (FBS) in rat first somatosensory cortex (SI) after digit stimulation.Exp Brain Res. 2002 Aug;145(4):411-28. doi: 10.1007/s00221-002-1124-7. Epub 2002 Jul 3. Exp Brain Res. 2002. PMID: 12172653
-
Retinal influences induce bidirectional changes in the kinetics of N-methyl-D-aspartate receptor-mediated responses in striate cortex cells during postnatal development.Neuroscience. 2007 Sep 7;148(3):683-99. doi: 10.1016/j.neuroscience.2007.07.005. Epub 2007 Jul 12. Neuroscience. 2007. PMID: 17706364
-
Two developmental switches in GABAergic signalling: the K+-Cl- cotransporter KCC2 and carbonic anhydrase CAVII.J Physiol. 2005 Jan 1;562(Pt 1):27-36. doi: 10.1113/jphysiol.2004.077495. Epub 2004 Nov 4. J Physiol. 2005. PMID: 15528236 Free PMC article. Review.
-
The Case of the Disappearing Spindle Burst.Neural Plast. 2016;2016:8037321. doi: 10.1155/2016/8037321. Epub 2016 Mar 28. Neural Plast. 2016. PMID: 27119028 Free PMC article. Review.
Cited by
-
Cortical development, electroencephalogram rhythms, and the sleep/wake cycle.Biol Psychiatry. 2015 Jun 15;77(12):1071-8. doi: 10.1016/j.biopsych.2014.12.017. Epub 2014 Dec 24. Biol Psychiatry. 2015. PMID: 25680672 Free PMC article. Review.
-
Loss of sleep when it is needed most - Consequences of persistent developmental sleep disruption: A scoping review of rodent models.Neurobiol Sleep Circadian Rhythms. 2022 Dec 6;14:100085. doi: 10.1016/j.nbscr.2022.100085. eCollection 2023 May. Neurobiol Sleep Circadian Rhythms. 2022. PMID: 36567958 Free PMC article.
-
Developmental profiles of infant EEG: overlap with transient cortical circuits.Clin Neurophysiol. 2012 Aug;123(8):1502-11. doi: 10.1016/j.clinph.2011.11.264. Epub 2012 Feb 17. Clin Neurophysiol. 2012. PMID: 22341979 Free PMC article.
-
Developmental 'awakening' of primary motor cortex to the sensory consequences of movement.Elife. 2018 Dec 21;7:e41841. doi: 10.7554/eLife.41841. Elife. 2018. PMID: 30574868 Free PMC article.
-
Twitch-related and rhythmic activation of the developing cerebellar cortex.J Neurophysiol. 2015 Sep;114(3):1746-56. doi: 10.1152/jn.00284.2015. Epub 2015 Jul 8. J Neurophysiol. 2015. PMID: 26156383 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

