Structural basis of binding of fluorescent, site-specific dansylated amino acids to human serum albumin
- PMID: 20940056
- PMCID: PMC3073228
- DOI: 10.1016/j.jsb.2010.10.004
Structural basis of binding of fluorescent, site-specific dansylated amino acids to human serum albumin
Abstract
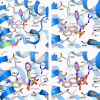
Human serum albumin (HSA) has two primary binding sites for drug molecules. These sites selectively bind different dansylated amino acid compounds, which-due to their intrinsic fluorescence-have long been used as specific markers for the drug pockets on HSA. We present here the co-crystal structures of HSA in complex with six dansylated amino acids that are specific for either drug site 1 (dansyl-l-asparagine, dansyl-l-arginine, dansyl-l-glutamate) or drug site 2 (dansyl-l-norvaline, dansyl-l-phenylalanine, dansyl-l-sarcosine). Our results explain the structural basis of the site-specificity of different dansylated amino acids. They also show that fatty acid binding has only a modest effect on binding of dansylated amino acids to drug site 1 and identify the location of secondary binding sites.
Copyright © 2010 Elsevier Inc. All rights reserved.
Figures


References
-
- Antolini L., Menabue L., Sola M., Battaglia L.P., Corradi A.B. The effect of a dansyl group on the co-ordinative ability of N-protected amino acids. Part 2. Binary copper(II) complexes and their pyridine and 2, 2′-bipyridine adducts. Crystal and molecular structure of the complexes aquabis (N-dansylglycinato-0)bis(pyridine)copper(11) and (2, 2′-bipyridine)-(N-dansylglycinato-NO)(methanol)copper(II), and neutral N-dansylglycine. J. Chem. Soc. Dalton Trans. 1986:1367–1373.
-
- Bhattacharya A.A., Curry S., Franks N.P. Binding of the general anesthetics propofol and halothane to human serum albumin: high-resolution crystal structures. J. Biol. Chem. 2000;275:38731–38738. - PubMed
-
- Bhattacharya A.A., Grüne T., Curry S. Crystallographic analysis reveals common modes of binding of medium and long-chain fatty acids to human serum albumin. J. Mol. Biol. 2000;303:721–732. - PubMed
-
- Birkett D.J., Myers S.P., Sudlow G. Effects of fatty acids on two specific drug binding sites on human serum albumin. Mol. Pharmacol. 1977;13:987–992. - PubMed
-
- Brodersen R. Binding of bilirubin to albumin. CRC Crit. Rev. Clin. Lab. Sci. 1980;11:305–399. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

