Phase III trial assessing bevacizumab in stages II and III carcinoma of the colon: results of NSABP protocol C-08
- PMID: 20940184
- PMCID: PMC3055856
- DOI: 10.1200/JCO.2010.30.0855
Phase III trial assessing bevacizumab in stages II and III carcinoma of the colon: results of NSABP protocol C-08
Abstract
Purpose: The National Surgical Adjuvant Breast and Bowel Project C-08 trial was designed to investigate the safety and efficacy of adding bevacizumab to modified FOLFOX6 (mFOLFOX6; ie, infusional/bolus fluorouracil, leucovorin, and oxaliplatin) for the adjuvant treatment of patients with stages II to III colon cancer.
Methods: Patients received mFOLFOX6 every 2 weeks for 26 weeks alone or modified as FOLFOX6 + bevacizumab (5 mg/kg every 2 weeks for 52 weeks [ie, experimental group]). The primary end point was disease-free survival (DFS).
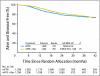
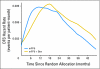
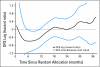
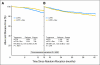
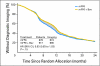
Results: Among 2,672 analyzed patients, demographic factors were well balanced by treatment. With a median follow-up of 35.6 months, the addition of bevacizumab to mFOLFOX6 did not result in an overall significant increase in DFS (hazard ratio [HR], 0.89; 95% CI, 0.76 to 1.04; P = .15). The point estimates for 3-year DFS for the overall population were 77.4% and 75.5% for the experimental and control arms, respectively. For patients with stages II and III diseases, these same estimates were 87.4% and 84.7%, respectively, for stage II and 74.2% and 72.4%, respectively, for stage III. Exploratory analyses found that the effect of bevacizumab on DFS was different before and after a 15-month landmark (time-by-treatment interaction P value < .0001). Bevacizumab had a strong effect before the landmark (HR, 0.61; 95% CI, 0.48 to 0.78; P < .001) but no significant effect after (HR, 1.22; 95% CI, 0.98 to 1.52; P = .076).
Conclusion: Bevacizumab for 1 year with mFOLFOX6 does not significantly prolong DFS in stages II and III colon cancer. However, a significant but transient effect during bevacizumab exposure was observed in the experimental arm. We postulate that this observation reflects a biologic effect during bevacizumab exposure. Given the lack of improvement in DFS, the use of bevacizumab cannot be recommended for use in the adjuvant treatment of patients with colon cancer.
Conflict of interest statement
Authors' disclosures of potential conflicts of interest and author contributions are found at the end of this article.
Figures


Comment in
-
Lessons from the adjuvant bevacizumab trial on colon cancer: what next?J Clin Oncol. 2011 Jan 1;29(1):1-4. doi: 10.1200/JCO.2010.32.2701. Epub 2010 Nov 29. J Clin Oncol. 2011. PMID: 21115866 No abstract available.
References
-
- André T, Boni C, Mounedji-Boudiaf L, et al. Oxaliplatin, fluorouracil, and leucovorin as adjuvant treatment for colon cancer. N Engl J Med. 2004;350:2343–2351. - PubMed
-
- Kuebler JP, Wieand HS, O'Connell MJ, et al. Oxaliplatin combined with weekly bolus fluorouracil and leucovorin as surgical adjuvant chemotherapy for stage II and III colon cancer: Results from NSABP C-07. J Clin Oncol. 2007;25:2198–2204. - PubMed
-
- Hurwitz H, Fehrenbacher L, Novotny W, et al. Bevacizumab plus irinotecan, fluorouracil, and leucovorin for metastatic colorectal cancer. N Engl J Med. 2004;350:2335–2342. - PubMed
-
- Saltz LB, Clarke S, Díaz-Rubio E, et al. Bevacizumab in combination with oxaliplatin-based chemotherapy as first-line therapy in metastatic colorectal cancer: A randomized phase III study. J Clin Oncol. 2008:2013–2019. - PubMed
-
- Ellis LM, Hicklin DJ. VEGF-targeted therapy: Mechanisms of anti-tumour activity. Nat Rev Cancer. 2008;8:579–591. - PubMed
Publication types
MeSH terms
Substances
Supplementary concepts
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

