Activity of IPI-504, a novel heat-shock protein 90 inhibitor, in patients with molecularly defined non-small-cell lung cancer
- PMID: 20940188
- PMCID: PMC4676802
- DOI: 10.1200/JCO.2010.30.8338
Activity of IPI-504, a novel heat-shock protein 90 inhibitor, in patients with molecularly defined non-small-cell lung cancer
Abstract
Purpose: IPI-504 is a novel, water-soluble, potent inhibitor of heat-shock protein 90 (Hsp90). Its potential anticancer activity has been validated in preclinical in vitro and in vivo models. We studied the activity of IPI-504 after epidermal growth factor receptor (EGFR) tyrosine kinase inhibitor (TKI) therapy in patients with advanced, molecularly defined non-small-cell lung cancer (NSCLC).
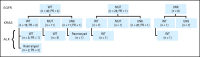
Patients and methods: Patients with advanced NSCLC, prior treatment with EGFR TKIs, and tumor tissue available for molecular genotyping were enrolled in this prospective, nonrandomized, multicenter, phase II study of IPI-504 monotherapy. The primary outcome was objective response rate (ORR). Secondary aims included safety, progression-free survival (PFS), and analysis of activity by molecular subtypes.
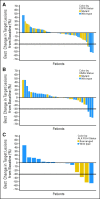
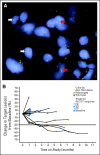
Results: Seventy-six patients were enrolled between December 2007 and May 2009 from 10 United States cancer centers. An ORR of 7% (five of 76) was observed in the overall study population, 10% (four of 40) in patients who were EGFR wild-type, and 4% (one of 28) in those with EGFR mutations. Although both EGFR groups were below the target ORR of 20%, among the three patients with an ALK gene rearrangement, two had partial responses and the third had prolonged stable disease (7.2 months, 24% reduction in tumor size). The most common adverse events included grades 1 and 2 fatigue, nausea, and diarrhea. Grade 3 or higher liver function abnormalities were observed in nine patients (11.8%).
Conclusion: IPI-504 has clinical activity in patients with NSCLC, particularly among patients with ALK rearrangements.
Conflict of interest statement
Authors' disclosures of potential conflicts of interest and author contributions are found at the end of this article.
Figures

References
-
- Whitesell L, Lindquist SL. HSP90 and the chaperoning of cancer. Nat Rev Cancer. 2005;5:761–772. - PubMed
-
- Xu W, Neckers L. Targeting the molecular chaperone heat shock protein 90 provides a multifaceted effect on diverse cell signaling pathways of cancer cells. Clin Cancer Res. 2007;13:1625–1629. - PubMed
-
- Workman P, Burrows F, Neckers L, et al. Drugging the cancer chaperone HSP90: Combinatorial therapeutic exploitation of oncogene addiction and tumor stress. Ann N Y Acad Sci. 2007;1113:202–216. - PubMed
-
- Rutherford SL, Lindquist S. Hsp90 as a capacitor for morphological evolution. Nature. 1998;396:336–342. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

