Atrial fibrillation and stroke associated with intravenous bisphosphonate therapy in older patients with cancer
- PMID: 20940190
- PMCID: PMC3020695
- DOI: 10.1200/JCO.2010.28.7524
Atrial fibrillation and stroke associated with intravenous bisphosphonate therapy in older patients with cancer
Abstract
Purpose: Recent studies have linked the use of intravenous and orally administered bisphosphonates with subsequent development of atrial fibrillation. Patients with cancer who receive intravenous bisphosphonate therapy may be at particular risk for this adverse event because they receive higher doses of these drugs than do patients treated for other indications. We examined the association of intravenous bisphosphonates with atrial fibrillation, all classifications of supraventricular tachycardia (SVT), and stroke among older patients with cancer.
Patients and methods: Using Surveillance, Epidemiology, and End Results (SEER)-Medicare-linked data, we identified older (≥ age 65 years) patients with cancer who were treated with intravenous infusions of bisphosphonates between January 1, 1995 and December 31, 2003. We then matched 13,714 bisphosphonate nonusers to 6,857 bisphosphonate users, at a 2:1 ratio, on cancer type, age, sex, presence of bone metastases, and SEER geographic region. Patients were observed until December 31, 2003 or until they lost coverage from Medicare Parts A and B; enrolled in a health maintenance organization; received a diagnosis of atrial fibrillation, any SVT, or stroke; or died.
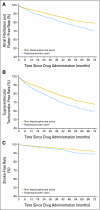
Results: Receipt of intravenous bisphosphonates was modestly associated with an increased risk for atrial fibrillation (hazard ratio [HR] = 1.30; 95% CI, 1.18 to 1.43), all SVT (HR = 1.28; 95% CI, 1.19 to 1.38), and stroke (HR = 1.30; 95% CI, 1.09 to 1.54). The risk for all SVT increased 7% for each increase of five bisphosphonate dose equivalents (HR = 1.07; 95% CI, 1.02 to 1.12).
Conclusion: Clinicians who treat patients with cancer who have received intravenous bisphosphonates should be aware of the possible cardiovascular adverse events associated with this treatment.
Conflict of interest statement
Authors' disclosures of potential conflicts of interest and author contributions are found at the end of this article.
Figures
Comment in
-
Bisphosphonate risks and benefits: finding a balance.J Clin Oncol. 2010 Nov 20;28(33):4873-6. doi: 10.1200/JCO.2010.31.1464. Epub 2010 Oct 12. J Clin Oncol. 2010. PMID: 20940189 No abstract available.
Similar articles
-
Oral bisphosphonates and risk of ischemic stroke: a case-control study.Osteoporos Int. 2011 Jun;22(6):1773-9. doi: 10.1007/s00198-010-1395-y. Epub 2010 Oct 13. Osteoporos Int. 2011. PMID: 20945149
-
Use of bisphosphonate and risk of atrial fibrillation in older women with osteoporosis.Osteoporos Int. 2012 Jan;23(1):247-54. doi: 10.1007/s00198-011-1608-z. Epub 2011 Mar 24. Osteoporos Int. 2012. PMID: 21431993
-
Intravenous bisphosphonate therapy and inflammatory conditions or surgery of the jaw: a population-based analysis.J Natl Cancer Inst. 2007 Jul 4;99(13):1016-24. doi: 10.1093/jnci/djm025. Epub 2007 Jun 27. J Natl Cancer Inst. 2007. PMID: 17596574
-
Bisphosphonates and the risk of atrial fibrillation.Endokrynol Pol. 2011 Jan-Feb;62(1):93-6. Endokrynol Pol. 2011. PMID: 21365587 Review.
-
[Bisphosphonates and the risk of atrial fibrillation].Endokrynol Pol. 2011;62 Suppl 3:10-3. Endokrynol Pol. 2011. PMID: 22161981 Review. Polish.
Cited by
-
A phase II, multicentre trial evaluating the efficacy of de-escalated bisphosphonate therapy in metastatic breast cancer patients at low-risk of skeletal-related events.Breast Cancer Res Treat. 2014 Apr;144(3):615-24. doi: 10.1007/s10549-014-2906-x. Epub 2014 Mar 18. Breast Cancer Res Treat. 2014. PMID: 24638849 Free PMC article. Clinical Trial.
-
Intravenous bisphosphonate therapy and atrial fibrillation/flutter risk in cancer patients: a nationwide cohort study.Br J Cancer. 2011 Sep 27;105(7):881-3. doi: 10.1038/bjc.2011.338. Epub 2011 Aug 30. Br J Cancer. 2011. PMID: 21878939 Free PMC article.
-
Long-term risks and benefits of oral anticoagulation in atrial fibrillation patients with cancer: A report from the GLORIA-AF registry.Eur J Clin Invest. 2025 Feb;55(2):e14347. doi: 10.1111/eci.14347. Epub 2024 Nov 13. Eur J Clin Invest. 2025. PMID: 39538376 Free PMC article.
-
Are bone targeted agents still useful in times of immunotherapy? The SAKK 80/19 BTA pilot study.Bone Rep. 2024 Jul 23;22:101794. doi: 10.1016/j.bonr.2024.101794. eCollection 2024 Sep. Bone Rep. 2024. PMID: 39139592 Free PMC article.
-
Optimising the use of bone-targeted agents in patients with metastatic cancers: a practical guide for medical oncologists.Support Care Cancer. 2011 Nov;19(11):1687-96. doi: 10.1007/s00520-011-1230-9. Epub 2011 Jul 22. Support Care Cancer. 2011. PMID: 21785900 Review.
References
-
- Woo SB, Hellstein JW, Kalmar JR. Narrative [corrected] review: Bisphosphonates and osteonecrosis of the jaws. Ann Intern Med. 2006;144:753–761. - PubMed
-
- US Food and Drug Administration: Drugs@FDA. FDA approved drugs and products. www.accessdata.fda.gov/scripts/cder/drugsatfda.
-
- Black DM, Delmas PD, Eastell R, et al. Once-yearly zoledronic acid for treatment of postmenopausal osteoporosis. N Engl J Med. 2007;356:1809–1822. - PubMed
-
- Cummings SR, Schwartz AV, Black DM. Alendronate and atrial fibrillation. N Engl J Med. 2007;356:1895–1896. - PubMed
-
- Loke YK, Jeevanantham V, Singh S. Bisphosphonates and atrial fibrillation: Systematic review and meta-analysis. Drug Saf. 2009;32:219–228. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical