SHIP deficiency causes Crohn's disease-like ileitis
- PMID: 20940287
- PMCID: PMC3022365
- DOI: 10.1136/gut.2009.202283
SHIP deficiency causes Crohn's disease-like ileitis
Abstract
Background: Inflammatory bowel disease (IBD) can arise from genetic mutations that compromise intestinal epithelial cell integrity or immune regulation. SHIP has previously been shown to play a pivotal role in limiting the number of immunoregulatory cells and their function.
Aim: To determine whether SHIP plays a pivotal role in control of immune tolerance in the gut mucosa.
Methods: Gastrointestinal pathology was assessed in three separate strains of SHIP-deficient mice and their respective wild-type (WT) littermates. Gastrointestinal pathology was analysed in SHIP-deficient hosts reconstituted with WT haematopoietic cell grafts, and WT hosts reconstituted with SHIP-deficient haematopoietic cell grafts including whole splenocytes, purified T cells or natural killer (NK) cells. Major immune cell populations were also analysed in the small intestine of SHIP-deficient mice and WT controls.
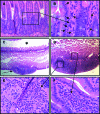
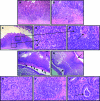
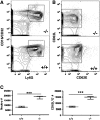
Results: SHIP-deficient mice developed segmental, transmural pyo-granulomatous ilietis that recapitulated classical features of Crohn's disease enteric pathology. Analysis of haematopoietic chimeras showed that WT bone marrow reconstitution of SHIP⁻/⁻ hosts corrects ileitis. Reconstitution with SHIP⁻/⁻ splenocytes transferred ileitis to WT hosts. Adoptive transfer of purified SHIP⁻/⁻ T cells or NK cells to WT hosts did not transfer ileitis. There was a paucity of both CD4 and CD8 T cells in the small intestines of SHIP-deficient mice; however, neutrophil numbers were significantly increased.
Conclusions: SHIP plays a pivotal role in immune function in the intestine; further scrutiny of this pathway in IBD patients is warranted. It is proposed that SHIP-deficient ileitis results from a local deficit in mucosal T cell immunity that promotes a damaging granulocyte-monocyte inflammation of the distal ileum.
Conflict of interest statement
Figures








Comment in
-
Intestinal expression of SHIP in inflammatory bowel diseases.Gut. 2012 Jun;61(6):956-7. doi: 10.1136/gutjnl-2011-301256. Epub 2011 Nov 3. Gut. 2012. PMID: 22052065 No abstract available.
References
-
- Sartor RB. Mechanisms of disease: pathogenesis of Crohn's disease and ulcerative colitis. Nat Clin Pract Gastroenterol Hepatol 2006;3:390–407 - PubMed
-
- Xavier RJ, Podolsky DK. Unravelling the pathogenesis of inflammatory bowel disease. Nature 2007;448:427–34 - PubMed
-
- Packey CD, Sartor RB. Interplay of commensal and pathogenic bacteria, genetic mutations, and immunoregulatory defects in the pathogenesis of inflammatory bowel diseases. J Intern Med 2008;263:597–606 - PubMed
-
- Cho JH. The genetics and immunopathogenesis of inflammatory bowel disease. Nat Rev Immunol 2008;8:458–66 - PubMed
-
- Wirtz S, Neurath MF. Mouse models of inflammatory bowel disease. Adv Drug Deliv Rev 2007;59:1073–83 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
