Negative regulation of EGFR-Vav2 signaling axis by Cbl ubiquitin ligase controls EGF receptor-mediated epithelial cell adherens junction dynamics and cell migration
- PMID: 20940296
- PMCID: PMC3013022
- DOI: 10.1074/jbc.M110.188086
Negative regulation of EGFR-Vav2 signaling axis by Cbl ubiquitin ligase controls EGF receptor-mediated epithelial cell adherens junction dynamics and cell migration
Abstract
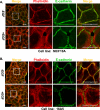
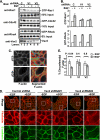
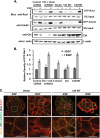
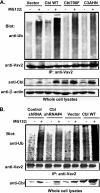
The E3 ubiquitin ligase Casitas B lymphoma protein (Cbl) controls the ubiquitin-dependent degradation of EGF receptor (EGFR), but its role in regulating downstream signaling elements with which it associates and its impact on biological outcomes of EGFR signaling are less clear. Here, we demonstrate that stimulation of EGFR on human mammary epithelial cells disrupts adherens junctions (AJs) through Vav2 and Rac1/Cdc42 activation. In EGF-stimulated cells, Cbl regulates the levels of phosphorylated Vav2 thereby attenuating Rac1/Cdc42 activity. Knockdown of Cbl and Cbl-b enhanced the EGF-induced disruption of AJs and cell motility. Overexpression of constitutively active Vav2 activated Rac1/Cdc42 and reorganized junctional actin cytoskeleton; these effects were suppressed by WT Cbl and enhanced by a ubiquitin ligase-deficient Cbl mutant. Cbl forms a complex with phospho-EGFR and phospho-Vav2 and facilitates phospho-Vav2 ubiquitinylation. Cbl can also interact with Vav2 directly in a Cbl Tyr-700-dependent manner. A ubiquitin ligase-deficient Cbl mutant enhanced the morphological transformation of mammary epithelial cells induced by constitutively active Vav2; this effect requires an intact Cbl Tyr-700. These results indicate that Cbl ubiquitin ligase plays a critical role in the maintenance of AJs and suppression of cell migration through down-regulation of EGFR-Vav2 signaling.
Figures





References
-
- Wells A. (1999) Int. J. Biochem. Cell Biol. 31, 637–643 - PubMed
-
- Yarden Y., Sliwkowski M. X. (2001) Nat. Rev. Mol. Cell Biol. 2, 127–137 - PubMed
-
- Arteaga C. (2003) Semin. Oncol. 30, Suppl. 7, 3–14 - PubMed
-
- Jost M., Kari C., Rodeck U. (2000) Eur. J. Dermatol. 10, 505–510 - PubMed
-
- Yarden Y. (2001) Eur. J. Cancer 37,S3–S8 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

