Protein kinase C phosphorylation regulates membrane insertion of GABAA receptor subtypes that mediate tonic inhibition
- PMID: 20940303
- PMCID: PMC3009907
- DOI: 10.1074/jbc.M110.149229
Protein kinase C phosphorylation regulates membrane insertion of GABAA receptor subtypes that mediate tonic inhibition
Abstract
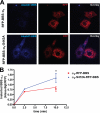
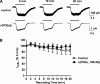
Tonic inhibition in the brain is mediated largely by specialized populations of extrasynaptic receptors, γ-aminobutyric acid receptors (GABA(A)Rs). In the dentate gyrus region of the hippocampus, tonic inhibition is mediated primarily by GABA(A)R subtypes assembled from α4β2/3 with or without the δ subunit. Although the gating of these receptors is subject to dynamic modulation by agents such as anesthetics, barbiturates, and neurosteroids, the cellular mechanisms neurons use to regulate their accumulation on the neuronal plasma membrane remain to be determined. Using immunoprecipitation coupled with metabolic labeling, we demonstrate that the α4 subunit is phosphorylated at Ser(443) by protein kinase C (PKC) in expression systems and hippocampal slices. In addition, the β3 subunit is phosphorylated on serine residues 408/409 by PKC activity, whereas the δ subunit did not appear to be a PKC substrate. We further demonstrate that the PKC-dependent increase of the cell surface expression of α4 subunit-containing GABA(A)Rs is dependent on Ser(443). Mechanistically, phosphorylation of Ser(443) acts to increase the stability of the α4 subunit within the endoplasmic reticulum, thereby increasing the rate of receptor insertion into the plasma membrane. Finally, we show that phosphorylation of Ser(443) increases the activity of α4 subunit-containing GABA(A)Rs by preventing current run-down. These results suggest that PKC-dependent phosphorylation of the α4 subunit plays a significant role in enhancing the cell surface stability and activity of GABA(A)R subtypes that mediate tonic inhibition.
Figures









References
-
- Farrant M., Nusser Z. (2005) Nat. Rev. Neurosci. 6, 215–229 - PubMed
-
- Rudolph U., Crestani F., Möhler H. (2001) Trends Pharmacol. Sci. 22, 188–194 - PubMed
-
- Sieghart W., Sperk G. (2002) Curr. Top. Med. Chem. 2, 795–816 - PubMed
-
- Brünig I., Scotti E., Sidler C., Fritschy J. M. (2002) J. Comp. Neurol. 443, 43–55 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

