Pivotal advance: The promotion of soluble DC-SIGN release by inflammatory signals and its enhancement of cytomegalovirus-mediated cis-infection of myeloid dendritic cells
- PMID: 20940323
- PMCID: PMC7166666
- DOI: 10.1189/jlb.0710386
Pivotal advance: The promotion of soluble DC-SIGN release by inflammatory signals and its enhancement of cytomegalovirus-mediated cis-infection of myeloid dendritic cells
Abstract
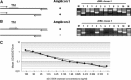
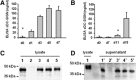
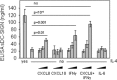
DC-SIGN is a member of the C-type lectin family. Mainly expressed by myeloid DCs, it is involved in the capture and internalization of pathogens, including human CMV. Several transcripts have been identified, some of which code for putative soluble proteins. However, little is known about the regulation and the functional properties of such putative sDC-SIGN variants. To better understand how sDC-SIGN could be involved in CMV infection, we set out to characterize biochemical and functional properties of rDC-SIGN as well as naturally occurring sDC-SIGN. We first developed a specific, quantitative ELISA and then used it to detect the presence sDC-SIGN in in vitro-generated DC culture supernatants as cell-free secreted tetramers. Next, in correlation with their inflammatory status, we demonstrated the presence of sDC-SIGN in several human body fluids, including serum, joint fluids, and BALs. CMV infection of human tissues was also shown to promote sDC-SIGN release. Based on the analysis of the cytokine/chemokine content of sDC-SIGN culture supernatants, we identified IFN-γ and CXCL8/IL-8 as inducers of sDC-SIGN production by MoDC. Finally, we demonstrated that sDC-SIGN was able to interact with CMV gB under native conditions, leading to a significant increase in MoDC CMV infection. Overall, our results confirm that sDC-SIGN, like its well-known, counterpart mDC-SIGN, may play a pivotal role in CMV-mediated pathogenesis.
Figures




Comment in
-
Editorial: How to play tag? DC-SIGN shows the way!J Leukoc Biol. 2011 Mar;89(3):321-3. doi: 10.1189/jlb.1010547. J Leukoc Biol. 2011. PMID: 21357245 No abstract available.
References
-
- Geijtenbeek, T. B. , Kwon, D. S. , Torensma, R. , van Vliet, S. J. , van Duijnhoven, G. C. , Middel, J. , Cornelissen, I. L. , Nottet, H. S. , KewalRamani, V. N. , Littman, D. R. , Figdor, C. G. , van Kooyk, Y. (2000) DC‐SIGN, a dendritic cell‐specific HIV‐1‐binding protein that enhances trans‐infection of T cells. Cell 100, 587–597. - PubMed
-
- Tailleux, L. , Pham‐Thi, N. , Bergeron‐Lafaurie, A. , Herrmann, J‐L. , Charles, P. , Schwartz, O. , Scheinmann, P. , Lagrange, P. H. , de Blic, J. , Tazi, A. , Gicquel, B. , Neyrolles, O. (2005) DC‐SIGN induction in alveolar macrophages defines privileged target host cells for mycobacteria in patients with tuberculosis. PLoS Med. 2, e381. - PMC - PubMed
-
- Van Lent, P. L. E. M. , Figdor, C. G. , Barrera, P. , van Ginkel, K. , Slöetjes, A. , van den Berg, W. B. , Torensma, R. (2003) Expression of the dendritic cell‐associated C‐type lectin DC‐SIGN by inflammatory matrix metallopro‐teinase‐producing macrophages in rheumatoid arthritis synovium and interaction with intercellular adhesion molecule 3‐positive T cells. Arthritis Rheum. 48, 360–369. - PubMed
-
- Feinberg, H. , Guo, Y. , Mitchell, D. A. , Drickamer, K. , Weis, W. I. (2005) Extended neck regions stabilize tetramers of the receptors DC‐SIGN and DC‐SIGNR. J. Biol. Chem. 280, 1327–1335. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

