Striatal and cortical BOLD, blood flow, blood volume, oxygen consumption, and glucose consumption changes in noxious forepaw electrical stimulation
- PMID: 20940730
- PMCID: PMC3063626
- DOI: 10.1038/jcbfm.2010.173
Striatal and cortical BOLD, blood flow, blood volume, oxygen consumption, and glucose consumption changes in noxious forepaw electrical stimulation
Abstract
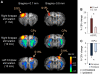
Recent reports showed noxious forepaw stimulation in rats evoked an unexpected sustained decrease in cerebral blood volume (CBV) in the bilateral striatum, whereas increases in spike activity and Fos-immunoreactive cells were observed. This study aimed to further evaluate the hemodynamic and metabolic needs in this model and the sources of negative functional magnetic resonance imaging (fMRI) signals by measuring blood oxygenation-level-dependent (BOLD), cerebral-blood-flow (CBF), CBV, and oxygen-consumption (i.e., cerebral metabolic rate of oxygen (CMRO(2))) changes using an 11.7-T MRI scanner, and glucose-consumption (i.e., cerebral metabolic rate of glucose (CMRglc)) changes using micro-positron emission tomography. In the contralateral somatosensory cortex, BOLD, CBF, CBV, CMRO(2) (n=7, P<0.05), and CMRglc (n=5, P<0.05) increased. In contrast, in the bilateral striatum, BOLD, CBF, and CBV decreased (P<0.05), CMRO(2) decreased slightly, although not significantly from baseline, and CMRglc was not statistically significant from baseline (P>0.05). These multimodal functional imaging findings corroborate the unexpected negative hemodynamic changes in the striatum during noxious forepaw stimulation, and support the hypothesis that striatal hemodynamic response is dominated by neurotransmitter-mediated vasoconstriction, overriding the stimulus-evoked fMRI signal increases commonly accompany elevated neuronal activity. Multimodal functional imaging approach offers a means to probe the unique attributes of the striatum, providing novel insights into the neurovascular coupling in the striatum. These findings may have strong implications in fMRI studies of pain.
Figures




References
-
- Chen CC, Shih YY, Mo KC, Yao NW, Lin ZJ, Huang CH, Shyu BC, Chang C. Mapping dopaminergic denervation in Parkinson disease in vivo and in situ: the visualization of structural details by CBV-weighted fMRI. J Cereb Blood Flow Metab. 2009;29:S607–S608.
-
- Choi JK, Chen YI, Hamel E, Jenkins BG. Brain hemodynamic changes mediated by dopamine receptors: role of the cerebral microvasculature in dopamine-mediated neurovascular coupling. NeuroImage. 2006;30:700–712. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

