Automated layer segmentation of macular OCT images using dual-scale gradient information
- PMID: 20941025
- PMCID: PMC3101081
- DOI: 10.1364/OE.18.021293
Automated layer segmentation of macular OCT images using dual-scale gradient information
Abstract
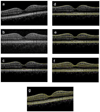
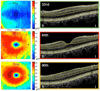
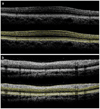
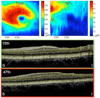
A novel automated boundary segmentation algorithm is proposed for fast and reliable quantification of nine intra-retinal boundaries in optical coherence tomography (OCT) images. The algorithm employs a two-step segmentation schema based on gradient information in dual scales, utilizing local and complementary global gradient information simultaneously. A shortest path search is applied to optimize the edge selection. The segmentation algorithm was validated with independent manual segmentation and a reproducibility study. It demonstrates high accuracy and reproducibility in segmenting normal 3D OCT volumes. The execution time is about 16 seconds per volume (480x512x128 voxels). The algorithm shows potential for quantifying images from diseased retinas as well.
Figures




Similar articles
-
Real-Time Automatic Segmentation of Optical Coherence Tomography Volume Data of the Macular Region.PLoS One. 2015 Aug 10;10(8):e0133908. doi: 10.1371/journal.pone.0133908. eCollection 2015. PLoS One. 2015. PMID: 26258430 Free PMC article.
-
Measurement of the Inner Macular Layers for Monitoring of Glaucoma: Confounding Effects of Age-Related Macular Degeneration.Ophthalmol Glaucoma. 2023 Jan-Feb;6(1):68-77. doi: 10.1016/j.ogla.2022.06.006. Epub 2022 Jun 21. Ophthalmol Glaucoma. 2023. PMID: 35750324 Free PMC article.
-
Intraretinal layer segmentation of macular optical coherence tomography images using optimal 3-D graph search.IEEE Trans Med Imaging. 2008 Oct;27(10):1495-505. doi: 10.1109/TMI.2008.923966. IEEE Trans Med Imaging. 2008. PMID: 18815101 Free PMC article.
-
Macular assessment using optical coherence tomography for glaucoma diagnosis.Br J Ophthalmol. 2012 Dec;96(12):1452-5. doi: 10.1136/bjophthalmol-2012-301845. Epub 2012 Sep 27. Br J Ophthalmol. 2012. PMID: 23018425 Free PMC article. Review.
-
Optical coherence tomography in imaging of macular diseases.Klin Oczna. 2010;112(4-6):138-46. Klin Oczna. 2010. PMID: 20825070 Review.
Cited by
-
Detecting glaucoma with visual fields derived from frequency-domain optical coherence tomography.Invest Ophthalmol Vis Sci. 2013 May 7;54(5):3289-96. doi: 10.1167/iovs.13-11639. Invest Ophthalmol Vis Sci. 2013. PMID: 23599332 Free PMC article.
-
Quantitative biometry of zebrafish retinal vasculature using optical coherence tomographic angiography.Biomed Opt Express. 2018 Feb 20;9(3):1244-1255. doi: 10.1364/BOE.9.001244. eCollection 2018 Mar 1. Biomed Opt Express. 2018. PMID: 29541517 Free PMC article.
-
Metabolic fingerprinting on retinal pigment epithelium thickness for individualized risk stratification of type 2 diabetes mellitus.Nat Commun. 2023 Oct 18;14(1):6573. doi: 10.1038/s41467-023-42404-1. Nat Commun. 2023. PMID: 37852995 Free PMC article.
-
Method for comparing visual field defects to local RNFL and RGC damage seen on frequency domain OCT in patients with glaucoma.Biomed Opt Express. 2011 Apr 5;2(5):1097-105. doi: 10.1364/BOE.2.001097. Biomed Opt Express. 2011. PMID: 21559122 Free PMC article.
-
Macular imaging with optical coherence tomography in glaucoma.Surv Ophthalmol. 2020 Nov-Dec;65(6):597-638. doi: 10.1016/j.survophthal.2020.03.002. Epub 2020 Mar 19. Surv Ophthalmol. 2020. PMID: 32199939 Free PMC article. Review.
References
-
- Fecher AF, Hitzenberger CK, Kamp G, Elzaizt SY. Measurement of intraocular distances by backscattering spectral interferometry. Opt. Commun. 1995;117(1–2):43–48.
-
- Wojtkowski M, Bajraszewski T, Targowski P, Kowalczyk A. Real-time in vivo imaging by high-speed spectral optical coherence tomography. Opt. Lett. 2003;28(19):1745–1747. - PubMed
-
- Cense B, Nassif N, Chen T, Pierce M, Yun SH, Park B, Bouma B, Tearney G, de Boer J. Ultrahigh-resolution high-speed retinal imaging using spectral-domain optical coherence tomography. Opt. Express. 2004;12(11):2435–2447. - PubMed
-
- Drexler W, Fujimoto JG. State-of-the-art retinal optical coherence tomography. Prog. Retin. Eye Res. 2008;27(1):45–88. - PubMed
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

