Aminoglycoside-free interventional antibiotic management in patients undergoing haemopoietic stem cell transplantation
- PMID: 20941340
- PMCID: PMC2951098
- DOI: 10.3205/dgkh000149
Aminoglycoside-free interventional antibiotic management in patients undergoing haemopoietic stem cell transplantation
Abstract
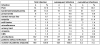
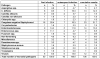
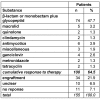
The position of aminoglycosides within interventional antibiosis in the early phase after stem cell transplantation has not been fully clarified so far although their use can induce serious renal impairment. To investigate this question early-infection data from 152 patients undergoing 195 allogeneic and autologous stem cell transplantations were investigated. Prophylaxis and treatment of infections followed international standards; however, aminoglycosides were omitted to avoid additional risks such as ototoxicity and nephrotoxicity and increased selection of resistant pathogens. Costs were another aspect.The overall-incidence of infections was 78% (152/195) and 67 patients showed more than one episode of infection. Fever of unknown origin and bacteriaemia/septicaemia dominated the spectrum of infections. The overall-response to interventional regimen consisting of β-lactam or carbapenem plus glycopeptides was 48%. Aminoglycosides were given in three patients in the late course of disease. Overall mortality was 15/195 (7.7%) and clearly related to infection in nine cases mostly due to mould infection. A comparison with previous published literature showed no hint for inferiority of 'aminoglycoside-free' antibiotic management in stem cell transplant patients. In conclusion, the present analysis supports the policy to omit aminoglycosides in the therapy of early infections in patients undergoing stem cell transplantation to avoid additional toxicity.
Die Bedeutung der Aminoglycoside für das empirische antibiotische Management in der Frühphase nach Stammzelltransplantation ist mit Ausnahme des Risikos schwerer Nierenschäden nicht vollständig geklärt. Zur Klärung dieser Frage wurden die Daten der Frühinfektion von 152 Patienten mit 195 allogener und autologer Stammzelltransplantation analysiert. Die Prophylaxe und Therapie der Infektionen erfolgte gemäß internationalen Standards mit der einzigen Ausnahme, dass auf den Einsatz von Aminoglycosiden verzichtet wurde, um ototoxische und nephrotoxische Risiken zu vermeiden und dem Selektionsdruck mit Verbreitung resistenter Erreger zu begegnen. Die Einsparung von Kosten war ein zusätzlicher Aspekt.
Die Inzidenz der Frühinfektion betrug 78% (152/195). 67 Patienten waren von mehr als einer Infektionsepisode betroffen, wobei Fieber unklarer Genese und Bakteriämie/Septikämie dominierten. Auf das interventionelle Regime mit β-Lactam oder Carbapenem + Glyopeptide reagierten 48% der Patientrn. Aminoglycoside wurden nur bei drei Patienten in der Spätphase der Erkrankung verabfolgt. Die Mortalität betrug 15/195 (7,7%) und war in neun Fällen eindeutig mit der Infektion, meist verursacht durch Schimmelpilze, assoziiert. Der Vergleich mit der Literatur ergibt keinen Anhalt für eine Unterlegenheit des Aminoglycosid-freien Antibiotika-Managements für Patienten mit Stammzelltransplantation. Damit stützt die vorliegende Studie die Möglichkeit des Verzichts auf Aminoglycoside in der Therapie der Frühinfektion von Patienten mit Stammzelltransplantation zur Vermeidung toxischer Risiken.
Keywords: conditioning therapy; high-dose therapy; infection; neutropenia; stem cell transplantation.
Figures



Similar articles
-
A clinical trial on efficacy and safety of teicoplanin in combination with beta-lactams and aminoglycosides in the treatment of severe sepsis of patients undergoing allogeneic/autologous bone marrow transplantation.Br J Haematol. 1990 Dec;76 Suppl 2:14-8. doi: 10.1111/j.1365-2141.1990.tb07929.x. Br J Haematol. 1990. PMID: 2149045 Clinical Trial.
-
Early infections in patients undergoing bone marrow or blood stem cell transplantation--a 7 year single centre investigation of 409 cases.Bone Marrow Transplant. 1999 Mar;23(6):589-97. doi: 10.1038/sj.bmt.1701614. Bone Marrow Transplant. 1999. PMID: 10217190
-
Extended-interval aminoglycoside administration for children: a meta-analysis.Pediatrics. 2004 Jul;114(1):e111-8. doi: 10.1542/peds.114.1.e111. Pediatrics. 2004. PMID: 15231982
-
β-lactam antibiotic versus combined β-lactam antibiotics and single daily dosing regimens of aminoglycosides for treating serious infections: A meta-analysis.Int J Antimicrob Agents. 2020 Mar;55(3):105839. doi: 10.1016/j.ijantimicag.2019.10.020. Epub 2019 Nov 5. Int J Antimicrob Agents. 2020. PMID: 31704215
-
Liposomal amphotericin B: a review of its use as empirical therapy in febrile neutropenia and in the treatment of invasive fungal infections.Drugs. 2009;69(3):361-92. doi: 10.2165/00003495-200969030-00010. Drugs. 2009. PMID: 19275278 Review.
References
-
- Leather HL, Wingard JR. Infections following hematopoietic stem cell transplantation. Infect Dis Clin North Am. 2001;15(2):483–520. - PubMed
-
- Kolbe K, Domkin D, Derigs HG, Bhakdi S, Huber C, Aulitzky WE. Infectious complications during neutropenia subsequent to peripheral blood stem cell transplantation. Bone Marrow Transplant. 1997;19(2):143–147. - PubMed
-
- Kruger W, Russmann B, Kroger N, Salomon C, Ekopf N, Elsner HA, et al. Early infections in patients undergoing bone marrow or blood stem cell transplantation – a 7 year single centre investigation of 409 cases. Bone Marrow Transplant. 1999;23(6):589–597. - PubMed
-
- Link H, Bohme A, Cornely OA, Hoffken K, Kellner O, Kern WV, et al. Antimicrobial therapy of unexplained fever in neutropenic patients - guidelines of the Infectious Diseases Working Party (AGIHO) of the German Society of Hematology and Oncology (DGHO), Study Group Interventional Therapy of Unexplained Fever, Arbeitsgemeinschaft Supportivmassnahmen in der Onkologie (ASO) of the Deutsche Krebsgesellschaft (DKG-German Cancer Society) Ann Hematol. 2003;82 Suppl 2:S105–S117. - PubMed
-
- Zinner SH. Relevant aspects in the Infectious Diseases Society of America (IDSA) guidelines for the use of antimicrobial agents in neutropenic patients with unexplained fever. Int J Hematol. 1998;68 Suppl 1:S31–S34. - PubMed
LinkOut - more resources
Full Text Sources

