HapX-mediated adaption to iron starvation is crucial for virulence of Aspergillus fumigatus
- PMID: 20941352
- PMCID: PMC2947994
- DOI: 10.1371/journal.ppat.1001124
HapX-mediated adaption to iron starvation is crucial for virulence of Aspergillus fumigatus
Abstract
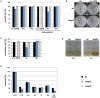
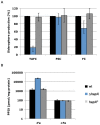
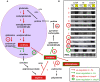
Iron is essential for a wide range of cellular processes. Here we show that the bZIP-type regulator HapX is indispensable for the transcriptional remodeling required for adaption to iron starvation in the opportunistic fungal pathogen Aspergillus fumigatus. HapX represses iron-dependent and mitochondrial-localized activities including respiration, TCA cycle, amino acid metabolism, iron-sulfur-cluster and heme biosynthesis. In agreement with the impact on mitochondrial metabolism, HapX-deficiency decreases resistance to tetracycline and increases mitochondrial DNA content. Pathways positively affected by HapX include production of the ribotoxin AspF1 and siderophores, which are known virulence determinants. Iron starvation causes a massive remodeling of the amino acid pool and HapX is essential for the coordination of the production of siderophores and their precursor ornithine. Consistent with HapX-function being limited to iron depleted conditions and A. fumigatus facing iron starvation in the host, HapX-deficiency causes significant attenuation of virulence in a murine model of aspergillosis. Taken together, this study demonstrates that HapX-dependent adaption to conditions of iron starvation is crucial for virulence of A. fumigatus.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures
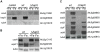
References
-
- Weinberg ED. The role of iron in protozoan and fungal infectious diseases. J Eukaryot Microbiol. 1999;46:231–238. - PubMed
-
- Weiss G. Iron and immunity: a double-edged sword. Eur J Clin Invest. 2002;32(Suppl 1):70–78. - PubMed
-
- Tekaia F, Latge JP. Aspergillus fumigatus: saprophyte or pathogen? Curr Opin Microbiol. 2005;8:385–392. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

