Pathogenic bacteria target NEDD8-conjugated cullins to hijack host-cell signaling pathways
- PMID: 20941356
- PMCID: PMC2947998
- DOI: 10.1371/journal.ppat.1001128
Pathogenic bacteria target NEDD8-conjugated cullins to hijack host-cell signaling pathways
Abstract
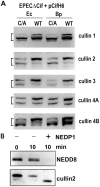
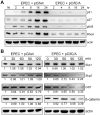
The cycle inhibiting factors (Cif), produced by pathogenic bacteria isolated from vertebrates and invertebrates, belong to a family of molecules called cyclomodulins that interfere with the eukaryotic cell cycle. Cif blocks the cell cycle at both the G₁/S and G₂/M transitions by inducing the stabilization of cyclin-dependent kinase inhibitors p21(waf1) and p27(kip1). Using yeast two-hybrid screens, we identified the ubiquitin-like protein NEDD8 as a target of Cif. Cif co-compartmentalized with NEDD8 in the host cell nucleus and induced accumulation of NEDD8-conjugated cullins. This accumulation occurred early after cell infection and correlated with that of p21 and p27. Co-immunoprecipitation revealed that Cif interacted with cullin-RING ubiquitin ligase complexes (CRLs) through binding with the neddylated forms of cullins 1, 2, 3, 4A and 4B subunits of CRL. Using an in vitro ubiquitylation assay, we demonstrate that Cif directly inhibits the neddylated CUL1-associated ubiquitin ligase activity. Consistent with this inhibition and the interaction of Cif with several neddylated cullins, we further observed that Cif modulates the cellular half-lives of various CRL targets, which might contribute to the pathogenic potential of diverse bacteria.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures




Similar articles
-
The bacterial effector Cif interferes with SCF ubiquitin ligase function by inhibiting deneddylation of Cullin1.Biochem Biophys Res Commun. 2010 Oct 15;401(2):268-74. doi: 10.1016/j.bbrc.2010.09.048. Epub 2010 Sep 17. Biochem Biophys Res Commun. 2010. PMID: 20850415
-
Cycle inhibiting factors (cifs): cyclomodulins that usurp the ubiquitin-dependent degradation pathway of host cells.Toxins (Basel). 2011 Apr;3(4):356-68. doi: 10.3390/toxins3040356. Epub 2011 Mar 29. Toxins (Basel). 2011. PMID: 22069713 Free PMC article. Review.
-
Inhibition of cullin RING ligases by cycle inhibiting factor: evidence for interference with Nedd8-induced conformational control.J Mol Biol. 2011 Oct 21;413(2):430-7. doi: 10.1016/j.jmb.2011.08.030. Epub 2011 Aug 29. J Mol Biol. 2011. PMID: 21903097
-
The cyclomodulin cycle inhibiting factor (CIF) alters cullin neddylation dynamics.J Biol Chem. 2013 May 24;288(21):14716-26. doi: 10.1074/jbc.M112.448258. Epub 2013 Apr 15. J Biol Chem. 2013. PMID: 23589306 Free PMC article.
-
Cif type III effector protein: a smart hijacker of the host cell cycle.Future Microbiol. 2009 Sep;4(7):867-77. doi: 10.2217/fmb.09.60. Future Microbiol. 2009. PMID: 19722840 Review.
Cited by
-
High prevalence of mucosa-associated E. coli producing cyclomodulin and genotoxin in colon cancer.PLoS One. 2013;8(2):e56964. doi: 10.1371/journal.pone.0056964. Epub 2013 Feb 14. PLoS One. 2013. PMID: 23457644 Free PMC article.
-
NEDDylation is essential for Kaposi's sarcoma-associated herpesvirus latency and lytic reactivation and represents a novel anti-KSHV target.PLoS Pathog. 2015 Mar 20;11(3):e1004771. doi: 10.1371/journal.ppat.1004771. eCollection 2015 Mar. PLoS Pathog. 2015. PMID: 25794275 Free PMC article.
-
Structure of a glomulin-RBX1-CUL1 complex: inhibition of a RING E3 ligase through masking of its E2-binding surface.Mol Cell. 2012 Aug 10;47(3):371-82. doi: 10.1016/j.molcel.2012.05.044. Epub 2012 Jun 28. Mol Cell. 2012. PMID: 22748924 Free PMC article.
-
What a difference a Dalton makes: bacterial virulence factors modulate eukaryotic host cell signaling systems via deamidation.Microbiol Mol Biol Rev. 2013 Sep;77(3):527-39. doi: 10.1128/MMBR.00013-13. Microbiol Mol Biol Rev. 2013. PMID: 24006474 Free PMC article. Review.
-
Proximity biotinylation at the host-Shigella interface reveals UFMylation as an antibacterial pathway.bioRxiv [Preprint]. 2025 May 29:2025.05.29.656827. doi: 10.1101/2025.05.29.656827. bioRxiv. 2025. PMID: 40501833 Free PMC article. Preprint.
References
-
- Samba-Louaka A, Taieb F, Nougayrede JP, Oswald E. Cif type III effector protein: a smart hijacker of the host cell cycle. Future Microbiol. 2009;4:867–877. - PubMed
-
- Nougayrede JP, Taieb F, De Rycke J, Oswald E. Cyclomodulins: bacterial effectors that modulate the eukaryotic cell cycle. Trends Microbiol. 2005;13:103–110. - PubMed
-
- Oswald E, Nougayrede JP, Taieb F, Sugai M. Bacterial toxins that modulate host cell-cycle progression. Curr Opin Microbiol. 2005;8:83–91. - PubMed
-
- Marches O, Ledger TN, Boury M, Ohara M, Tu X, et al. Enteropathogenic and enterohaemorrhagic Escherichia coli deliver a novel effector called Cif, which blocks cell cycle G2/M transition. Mol Microbiol. 2003;50:1553–1567. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

