Aberrant cytoplasm localization and protein stability of SIRT1 is regulated by PI3K/IGF-1R signaling in human cancer cells
- PMID: 20941378
- PMCID: PMC2952410
- DOI: 10.7150/ijbs.6.599
Aberrant cytoplasm localization and protein stability of SIRT1 is regulated by PI3K/IGF-1R signaling in human cancer cells
Abstract
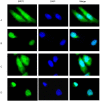
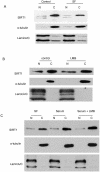
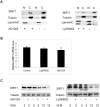
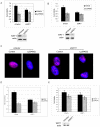
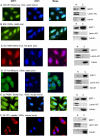
SIRT1, an NAD-dependent histone/protein deacetylase, has classically been thought of as a nuclear protein. In this study, we demonstrate that SIRT1 is mainly localized in the nucleus of normal cells, but is predominantly localized in the cytoplasm of the cancer / transformed cells we tested. We found this predominant cytoplasmic localization of SIRT1 is regulated by elevated mitotic activity and PI3K/IGF-1R signaling in cancer cells. We show that aberrant cytoplasmic localization of SIRT1 is due to increased protein stability and is regulated by PI3K/IGF-1R signaling. In addition, we determined that SIRT1 is required for PI3K-mediated cancer cell growth. Our study represents the first identification that aberrant cytoplasm localization is one of the specific alternations to SIRT1 that occur in cancer cells, and PI3K/IGF-1R signaling plays an important role in the regulation of cytoplasmic SIRT1 stability. Our findings suggest that the over-expressed cytoplasmic SIRT1 in cancer cells may greatly contribute to its cancer-specific function by working downstream of the PI3K/IGF-1R signaling pathway.
Keywords: PI3K/IGF-1R; SIRT1; cancer cells; cytoplasm localization; protein stability.
Conflict of interest statement
CONFLICTS OF INTEREST: The authors declare that they have no conflicts of interest
Figures

References
-
- Saunders L.R, Verdin E. Sirtuins: critical regulators at the crossroads between cancer and aging. Oncogene. 2007;26(37):5489–504. - PubMed
-
- Yamamoto H, Schoonjans K, Auwerx J. Sirtuin functions in health and disease. Mol Endocrinol. 2007;21(8):1745–55. - PubMed
-
- Zeng L, Chen R, Liang F. et al.Silent information regulator, Sirtuin 1, and age-related diseases. Geriatr Gerontol Int. 2009;9(1):7–15. - PubMed
-
- Donmez G, Guarente L. Aging and disease: connections to sirtuins. Aging Cell. 2010;9(2):285–90. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

