Computational studies of colicin insertion into membranes: the closed state
- PMID: 20941706
- PMCID: PMC2995820
- DOI: 10.1002/prot.22866
Computational studies of colicin insertion into membranes: the closed state
Abstract
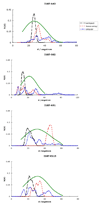
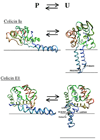
Colicins are water-soluble toxins that, upon interaction with membranes, undergo a conformational change, insert, and form pores in them. Pore formation activity is localized in a bundle of 10 α-helices named the pore-forming domain (PFD). There is evidence that colicins attach to the membrane via a hydrophobic hairpin embedded in the core of the PFD. Two main models have been suggested for the membrane-bound state: penknife and umbrella, differing in regard to the orientation of the hydrophobic hairpin with respect to the membrane. The arrangement of the amphipathic helices has been described as either a compact three-dimensional structure or a two-dimensional array of loosely interacting helices on the membrane surface. Using molecular dynamics simulations with an implicit membrane model, we studied the structure and stability of the conformations proposed earlier for four colicins. We find that colicins are initially driven towards the membrane by electrostatic interactions between basic residues and the negatively charged membrane surface. They do not have a unique binding orientation, but in the predominant orientations the central hydrophobic hairpin is parallel to the membrane. In the inserted state, the estimated free energy tends to be lower for the compact arrangements of the amphipathic helix, but the more expanded ones are in better agreement with experimental distance distributions. The difference in energy between penknife and umbrella conformations is small enough for equilibrium to exist between them. Elongation of the hydrophobic hairpin helices and membrane thinning were found unable to produce stabilization of the transmembrane configuration of the hydrophobic hairpin.
Copyright © 2010 Wiley-Liss, Inc.
Figures






Similar articles
-
A mechanism for toxin insertion into membranes is suggested by the crystal structure of the channel-forming domain of colicin E1.Structure. 1997 Mar 15;5(3):443-58. doi: 10.1016/s0969-2126(97)00200-1. Structure. 1997. PMID: 9083117
-
Conformation of the closed channel state of colicin A in proteoliposomes: an umbrella model.J Mol Biol. 2008 Apr 18;378(1):204-14. doi: 10.1016/j.jmb.2008.02.038. Epub 2008 Mar 4. J Mol Biol. 2008. PMID: 18353363
-
Membrane-bound state of the colicin E1 channel domain as an extended two-dimensional helical array.Proc Natl Acad Sci U S A. 1998 Apr 14;95(8):4282-7. doi: 10.1073/pnas.95.8.4282. Proc Natl Acad Sci U S A. 1998. PMID: 9539728 Free PMC article.
-
Insertion intermediates of pore-forming colicins in membrane two-dimensional space.Biochimie. 2002 May-Jun;84(5-6):465-75. doi: 10.1016/s0300-9084(02)01453-0. Biochimie. 2002. PMID: 12423790 Review.
-
Structure-function of the channel-forming colicins.Annu Rev Biophys Biomol Struct. 1995;24:611-41. doi: 10.1146/annurev.bb.24.060195.003143. Annu Rev Biophys Biomol Struct. 1995. PMID: 7545041 Review.
Cited by
-
Implicit membrane treatment of buried charged groups: application to peptide translocation across lipid bilayers.Biochim Biophys Acta. 2014 Sep;1838(9):2149-59. doi: 10.1016/j.bbamem.2014.01.015. Epub 2014 Feb 10. Biochim Biophys Acta. 2014. PMID: 24525075 Free PMC article.
-
Mechanism of negative membrane curvature generation by I-BAR domains.Structure. 2021 Dec 2;29(12):1440-1452.e4. doi: 10.1016/j.str.2021.07.010. Epub 2021 Sep 13. Structure. 2021. PMID: 34520736 Free PMC article.
References
-
- Lakey JH, Slatin SL. Pore forming toxins. In: Goot Gvd., editor. Pore-forming colicins and their relatives. Springer; 2001. 166 pp. - PubMed
-
- James R, Kleanthous C, Moore GR. The biology of E colicins: Paradigms and paradoxes. Microbiology-(UK) 1996;142:1569–1580. - PubMed
-
- Cramer WA, Heymann JB, Schendel SL, Deriy BN, Cohen FS, Elkins PA, Stauffacher CV. Structure-function of the channel-forming colicins. Annu Rev Biophys Biomol Struct. 1995;24:611–641. - PubMed
-
- Schein SJ, Kagan BL, Finkelstein A. Colicin-K acts by forming voltage-dependent channels in phospholipid bilayer membranes. Nature. 1978;276(5684):159–163. - PubMed
-
- Cleveland MV, Slatin S, Finkelstein A, Levinthal C. Structure-function-relationships for a voltage-dependent ion channel - Properties of COOH-terminal fragments of Colicin-E1. Proceedings of the National Academy of Sciences of the United States of America-Biological Sciences. 1983;80(12):3706–3710. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

