Evaluation of strain-induced hydrophobicity of pharmaceutical blends and its effect on drug release rate under multiple compression conditions
- PMID: 20942612
- PMCID: PMC4084706
- DOI: 10.3109/03639045.2010.521160
Evaluation of strain-induced hydrophobicity of pharmaceutical blends and its effect on drug release rate under multiple compression conditions
Abstract
Objective: The purpose of this study was to investigate the effect of mechanical shear on hydrophobicity of pharmaceutical powder blends as a function of composition and particle size, and to determine the impact on drug release from tablets.
Methods: Four powder formulations were subjected to three different shear strain conditions (40 rev, 160 rev, and 640 rev) in a controlled shear environment operating at a shear rate of 80 rpm. A total of 12 blends were tested for hydrophobicity. Subsequently, sheared blends were compressed into tablets at 8 kN and 12 kN in a rotary tablet press. During tablet compression, powder samples were collected after the feed frame and their hydrophobicity was again measured.
Results: Results indicated that increase in shear strain could significantly increase hydrophobicity, predominantly as an interacting function of blend composition. Blends with both colloidal silica and magnesium stearate (MgSt) were found to show higher hydrophobicity with shear than other blends. Additional shear applied by the tablet press feed frame was found to change the powder hydrophobicity only in the absence of MgSt.
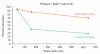
Conclusions: Studies showed that the drug release rates dropped with shear more for the blends with both colloidal silica and MgSt than the other blends. Furthermore, the rate of drug release dropped with a decrease in particle size of the main excipient. Surprisingly, the relationship between the relative increase in hydrophobicity and a corresponding drop in the drug release rate was not found when either MgSt or colloidal silica was mixed alone in the blends.
Figures



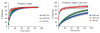



Similar articles
-
Mixing order of glidant and lubricant--influence on powder and tablet properties.Int J Pharm. 2011 May 16;409(1-2):269-77. doi: 10.1016/j.ijpharm.2011.02.032. Epub 2011 Feb 26. Int J Pharm. 2011. PMID: 21356286 Free PMC article.
-
Improved properties of fine active pharmaceutical ingredient powder blends and tablets at high drug loading via dry particle coating.Int J Pharm. 2018 May 30;543(1-2):288-299. doi: 10.1016/j.ijpharm.2018.04.002. Epub 2018 Apr 3. Int J Pharm. 2018. PMID: 29625168
-
Investigations on the Mechanism of Magnesium Stearate to Modify Aerosol Performance in Dry Powder Inhaled Formulations.J Pharm Sci. 2018 Apr;107(4):984-998. doi: 10.1016/j.xphs.2017.12.006. Epub 2017 Dec 14. J Pharm Sci. 2018. PMID: 29247741
-
A drop penetration method to measure powder blend wettability.Int J Pharm. 2018 Mar 1;538(1-2):112-118. doi: 10.1016/j.ijpharm.2017.12.034. Epub 2017 Dec 15. Int J Pharm. 2018. PMID: 29253584
-
Continuous direct tablet compression: effects of impeller rotation rate, total feed rate and drug content on the tablet properties and drug release.Drug Dev Ind Pharm. 2013 Nov;39(11):1802-8. doi: 10.3109/03639045.2012.738681. Epub 2012 Nov 19. Drug Dev Ind Pharm. 2013. PMID: 23163644
Cited by
-
Effect of Mixer Type on Particle Coating by Magnesium Stearate for Friction and Adhesion Modification.Pharmaceutics. 2021 Aug 5;13(8):1211. doi: 10.3390/pharmaceutics13081211. Pharmaceutics. 2021. PMID: 34452172 Free PMC article.
-
An investigation into the effects of excipient particle size, blending techniques and processing parameters on the homogeneity and content uniformity of a blend containing low-dose model drug.PLoS One. 2017 Jun 13;12(6):e0178772. doi: 10.1371/journal.pone.0178772. eCollection 2017. PLoS One. 2017. PMID: 28609454 Free PMC article.
-
Inhalable Mucociliary-On-Chip System Revealing Pulmonary Clearance Dynamics in Nanodrug Delivery.ACS Nano. 2025 Jan 21;19(2):2228-2244. doi: 10.1021/acsnano.4c11693. Epub 2025 Jan 7. ACS Nano. 2025. PMID: 39772499 Free PMC article.
-
Semi-mechanistic reduced order model of pharmaceutical tablet dissolution for enabling Industry 4.0 manufacturing systems.Int J Pharm. 2023 Jan 25;631:122502. doi: 10.1016/j.ijpharm.2022.122502. Epub 2022 Dec 16. Int J Pharm. 2023. PMID: 36529354 Free PMC article.
-
Mixing order of glidant and lubricant--influence on powder and tablet properties.Int J Pharm. 2011 May 16;409(1-2):269-77. doi: 10.1016/j.ijpharm.2011.02.032. Epub 2011 Feb 26. Int J Pharm. 2011. PMID: 21356286 Free PMC article.
References
-
- Genc L, Bilac H, Guler E. Studies on controlled release dimenhydrinate from matrix tablet formulations. Pharm Acta Helv. 1999;74:43–9. - PubMed
-
- Li JZ, Rekhi GS, Augsburger LL, Shangraw RF. The role of intra- and extragranular microcrystalline cellulose in tablet dissolution. Pharm Dev Tech. 1996;1:343–55. - PubMed
-
- Mehrotra A, Llusa M, Faqih A, Muzzio FJ. Influence of shear intensity and total shear on properties of blends and tablets of lactose and cellulose lubricated with magnesium stearate. Int J Pharm. 2007;336:284–91. - PubMed
-
- Safouane M, Langevin D, Binks BP. Effect of particle hydrophobicity on the properties of silica particle layers at the air-water interface. Langmuir. 2007;23:11546–53. - PubMed
-
- Sheskey PJ, Robb RT, Moore RD. Effects of lubricant level, method of mixing, and duration of mixing on a controlled-release matrix tablet containing hydroxypropyl methylcellulose. Drug Dev Ind Pharm. 1995;21:2151–65.
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
