A randomized factorial trial comparing 4 treatment regimens in treatment-naive HIV-infected persons with AIDS and/or a CD4 cell count <200 cells/μL in South Africa
- PMID: 20942650
- PMCID: PMC3008165
- DOI: 10.1086/656718
A randomized factorial trial comparing 4 treatment regimens in treatment-naive HIV-infected persons with AIDS and/or a CD4 cell count <200 cells/μL in South Africa
Abstract
Background: Few randomized trials comparing antiretroviral therapy (ART) regimens have been conducted in resource-limited settings.
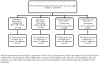
Methods: In the Republic of South Africa, antiretroviral-naive human immunodeficiency virus (HIV)-infected individuals >14 years old with a CD4 cell count <200 cells/μL or a prior AIDS diagnosis were randomized to receive efavirenz (EFV) or lopinavir/ritonavir (LPV/r) with either zidovudine (ZDV) plus didanosine (ddI) or stavudine (d4T) plus lamivudine (3TC) in an open-label, 2-by-2 factorial study and followed up for the primary outcome of AIDS or death and prespecified secondary outcomes, including CD4 cell count and viral load changes, treatment discontinuation, and grade 4 events.
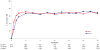
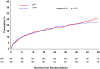
Results: In total, 1771 persons were randomized and followed up for a median of 24.7 months. AIDS or death occurred in (1) 163 participants assigned EFV and 157 assigned LPV/r (hazard ratio [HR], 1.04 [95% confidence interval {CI}, 0.84-1.30]) and in (2) 170 participants assigned ZDV+ddI and 150 assigned d4T+3TC (HR, 1.15 [95% CI, 0.93-1.44]). HIV RNA levels were lower (P < .001) and CD4 cell counts were greater (P < .01) over follow-up for d4T+3TC versus ZDV+ddI. Rates of potentially life-threatening adverse events and overall treatment discontinuation were similar for d4T+3TC and ZDV+ddI; however, more participants discontinued d4T because of toxicity (12.6%) than other treatments (<5%).
Conclusion: EFV and LPV/r are effective components of first-line ART. The poorer viral and immune responses with ZDV+ddI and the greater toxicity-associated discontinuation rate with d4T+3TC suggest that these treatments be used cautiously as initial therapy.
Trial registration: ClinicalTrials.gov identifier: NCT00342355.
Conflict of interest statement
Sean Emery has received grant support, travel awards and honoriums from Abbott, Boehnringer-Ingleheim, Bristol Myers Squibb, Gilead Sciences, GlaxoSmithKline, Merck, and Tibotec. All other authors do not have a commercial or other association that may pose a conflict of interest.
Figures






Comment in
-
Rescue of severely immunocompromised HIV-positive persons.J Infect Dis. 2010 Nov 15;202(10):1467-9. doi: 10.1086/656719. Epub 2010 Oct 13. J Infect Dis. 2010. PMID: 20942649 No abstract available.
References
-
- Joint United Nations Programme on HIV/AIDS., NetLibrary Inc. 2006 report on the global AIDS epidemic. Geneva: UNAIDS; 2006. A UNAIDS 10th anniversary special ed.
-
- United States. Dept. of Health and Human Services., National Institutes of Health (U.S.). Office of AIDS Research. Advisory Council. Guidelines for the use of antiretroviral agents in HIV-1-infected adults and adolescents: November 3, 2008. Washington, D.C: Dept. of Health and Human Services; 2008.
-
- Health SANDo. National Antiretroviral Treatment Guidelines. Johannesburg: Jacana; 2004.
-
- Joint United Nations Programme on HIV/AIDS., World Health Organization. Progress on global access to HIV antiretroviral therapy a report on “3 by 5”, June 2005 and beyond. Geneva: World Health Organization: UNAIDS; 2006.
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

