Antiretroviral therapies in women after single-dose nevirapine exposure
- PMID: 20942666
- PMCID: PMC2994321
- DOI: 10.1056/NEJMoa0906626
Antiretroviral therapies in women after single-dose nevirapine exposure
Abstract
Background: Peripartum administration of single-dose nevirapine reduces mother-to-child transmission of human immunodeficiency virus type 1 (HIV-1) but selects for nevirapine-resistant virus.
Methods: In seven African countries, women infected with HIV-1 whose CD4+ T-cell counts were below 200 per cubic millimeter and who either had or had not taken single-dose nevirapine at least 6 months before enrollment were randomly assigned to receive antiretroviral therapy with tenofovir–emtricitabine plus nevirapine or tenofovir-emtricitabine plus lopinavir boosted by a low dose of ritonavir. The primary end point was the time to confirmed virologic failure or death.
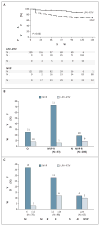
Results: A total of 241 women who had been exposed to single-dose nevirapine began the study treatments (121 received nevirapine and 120 received ritonavir-boosted lopinavir). Significantly more women in the nevirapine group reached the primary end point than in the ritonavir-boosted lopinavir group (26% vs. 8%) (adjusted P=0.001). Virologic failure occurred in 37 (28 in the nevirapine group and 9 in the ritonavir-boosted lopinavir group), and 5 died without prior virologic failure (4 in the nevirapine group and 1 in the ritonavir-boosted lopinavir group). The group differences appeared to decrease as the interval between single-dose nevirapine exposure and the start of antiretroviral therapy increased. Retrospective bulk sequencing of baseline plasma samples showed nevirapine resistance in 33 of 239 women tested (14%). Among 500 women without prior exposure to single-dose nevirapine, 34 of 249 in the nevirapine group (14%) and 36 of 251 in the ritonavir-boosted lopinavir group (14%) had virologic failure or died.
Conclusions: In women with prior exposure to peripartum single-dose nevirapine (but not in those without prior exposure), ritonavir-boosted lopinavir plus tenofovir–emtricitabine was superior to nevirapine plus tenofovir–emtricitabine for initial antiretroviral therapy. (Funded by the National Institute of Allergy and Infectious Diseases and the National Research Center; ClinicalTrials.gov number, NCT00089505.).
Conflict of interest statement
No other potential conflict of interest relevant to this article was reported.
Figures

Comment in
-
Preventing mother-to-child transmission of HIV—protecting this generation and the next.N Engl J Med. 2010 Oct 14;363(16):1570-2. doi: 10.1056/NEJMe1009863. N Engl J Med. 2010. PMID: 20942674 No abstract available.
References
-
- AIDS epidemic update. Geneva: UNAIDS; Nov, 2009.
-
- Towards universal access: scaling up priority HIV/AIDS interventions in the health sector: progress report 2008. Geneva: World Health Organization; 2008.
-
- Antiretroviral drugs for treating pregnant women and preventing HIV infection in infants recommendations for a public health approach — 2010 version. Geneva: World Health Organization; 2010. - PubMed
-
- Eshleman SH, Guay LA, Mwatha A, et al. Characterization of nevirapine resistance mutations in women with subtype A vs. D HIV-1 6–8 weeks after single-dose nevirapine (HIVNET 012) J Acquir Immune Defic Syndr. 2004;35:126–30. - PubMed
-
- Kassaye S, Lee E, Kantor R, et al. Drug resistance in plasma and breast milk after single-dose nevirapine in subtype C HIV type 1: population and clonal sequence analysis. AIDS Res Hum Retroviruses. 2007;23:1055–61. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
