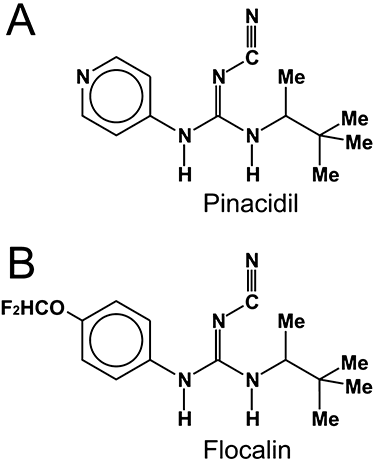
Sarcolemmal cardiac K(ATP) channels as a target for the cardioprotective effects of the fluorine-containing pinacidil analogue, flocalin
- PMID: 20942816
- PMCID: PMC3041258
- DOI: 10.1111/j.1476-5381.2010.01072.x
Sarcolemmal cardiac K(ATP) channels as a target for the cardioprotective effects of the fluorine-containing pinacidil analogue, flocalin
Abstract
Background and purpose: A class of drugs known as K(ATP) -channel openers induce cardioprotection. This study examined the effects of the novel K(ATP) -channel opener, the fluorine-containing pinacidil derivative, flocalin, on cardiac-specific K(ATP) -channels, excitability of native cardiac myocytes and on the ischaemic heart.
Experimental approach: The action of flocalin was investigated on: (i) membrane currents through cardiac-specific K(ATP) -channels (I(KATP) ) formed by K(IR) 6.2/SUR2A heterologously expressed in HEK-293 cells (HEK-293(₆.₂/₂A) ); (ii) excitability and intracellular Ca²(+) ([Ca²(+) ](i) ) transients of cultured rat neonatal cardiac myocytes; and (iii) functional and ultrastructural characteristics of isolated guinea-pig hearts subjected to ischaemia-reperfusion.
Key results: Flocalin concentration-dependently activated a glibenclamide-sensitive I(KATP) in HEK-293(₆.₂/₂A) cells with an EC₅₀= 8.1 ± 0.4 µM. In cardiac myocytes, flocalin (5 µM) hyperpolarized resting potential by 3-5 mV, markedly shortened action potential duration, reduced the amplitude of [Ca²(+) ](i) transients by 2-3-fold and suppressed contraction. The magnitude and extent of reversibility of these effects depended on the type of cardiac myocytes. In isolated hearts, perfusion with 5 µmol·L⁻¹ flocalin, before inducing ischaemia, facilitated restoration of contraction during reperfusion, decreased the number of extrasystoles, prevented the appearance of coronary vasoconstriction and reduced damage to the cardiac tissue at the ultrastructural level (state of myofibrils, membrane integrity, mitochondrial cristae structure).
Conclusion and implications: Flocalin induced potent cardioprotection by activating cardiac-type K(ATP) -channels with all the benefits of the presence of fluorine group in the drug structure: higher lipophilicity, decreased toxicity, resistance to oxidation and thermal degradation, decreased metabolism in the organism and prolonged therapeutic action.
© 2011 The Authors. British Journal of Pharmacology © 2011 The British Pharmacological Society.
Figures





References
-
- Begue J-P, Bonnet-Delpon D. Effects of fluorine substitution on biological properties. In: Begue J-P, Bonnet-Delpon D, editors. Bioorganic and Medicinal Chemistry of Fluorine. Hoboken, New Jersey: John Wiley & Sons, Inc.; 2008. pp. 72–98.
-
- Bhatnagar A, Bolli R. Modulation of KATP channels to protect the ischemic myocardium: clinical implications. Exp Clin Cardiol. 1999;4:20–22.
-
- Charnock JS. Omega-3 polyunsaturated fatty acids and ventricular fibrillation: the possible involvement of eicosanoids. Prostaglandins Leukot Essent Fatty Acids. 1999;61:243–247. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

