Differential expression of a BMP4 reporter allele in anterior fungiform versus posterior circumvallate taste buds of mice
- PMID: 20942907
- PMCID: PMC2966460
- DOI: 10.1186/1471-2202-11-129
Differential expression of a BMP4 reporter allele in anterior fungiform versus posterior circumvallate taste buds of mice
Abstract
Background: Bone Morphogenetic Protein 4 (BMP4) is a diffusible factor which regulates embryonic taste organ development. However, the role of BMP4 in taste buds of adult mice is unknown. We utilized transgenic mice with LacZ under the control of the BMP4 promoter to reveal the expression of BMP4 in the tongues of adult mice. Further we evaluate the pattern of BMP4 expression with that of markers of specific taste bud cell types and cell proliferation to define and compare the cell populations expressing BMP4 in anterior (fungiform papillae) and posterior (circumvallate papilla) tongue.
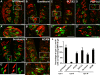
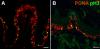
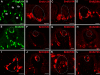
Results: BMP4 is expressed in adult fungiform and circumvallate papillae, i.e., lingual structures composed of non-taste epithelium and taste buds. Unexpectedly, we find both differences and similarities with respect to expression of BMP4-driven ß-galactosidase. In circumvallate papillae, many fusiform cells within taste buds are BMP4-ß-gal positive. Further, a low percentage of BMP4-expressing cells within circumvallate taste buds is immunopositive for markers of each of the three differentiated taste cell types (I, II and III). BMP4-positive intragemmal cells also expressed a putative marker of immature taste cells, Sox2, and consistent with this finding, intragemmal cells expressed BMP4-ß-gal within 24 hours after their final mitosis, as determined by BrdU birthdating. By contrast, in fungiform papillae, BMP4-ß-gal positive cells are never encountered within taste buds. However, in both circumvallate and fungiform papillae, BMP4-ß-gal expressing cells are located in the perigemmal region, comprising basal and edge epithelial cells adjacent to taste buds proper. This region houses the proliferative cell population that gives rise to adult taste cells. However, perigemmal BMP4-ß-gal cells appear mitotically silent in both fungiform and circumvallate taste papillae, as we do not find evidence of their active proliferation using cell cycle immunomarkers and BrdU birthdating.
Conclusion: Our data suggest that intragemmal BMP4-ß-gal cells in circumvallate papillae are immature taste cells which eventually differentiate into each of the 3 taste cell types, whereas perigemmal BMP4-ß-gal cells in both circumvallate and fungiform papillae may be slow cycling stem cells, or belong to the stem cell niche to regulate taste cell renewal from the proliferative cell population.
Figures





References
-
- Pumplin DW, Getschman E, Boughter JD Jr, Yu C, Smith DV. Differential expression of carbohydrate blood-group antigens on rat taste-bud cells: relation to the functional marker alpha-gustducin. J Comp Neurol. 1999;415(2):230–239. doi: 10.1002/(SICI)1096-9861(19991213)415:2<230::AID-CNE7>3.0.CO;2-Y. - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

