Evolutionary history of the poly(ADP-ribose) polymerase gene family in eukaryotes
- PMID: 20942953
- PMCID: PMC2964712
- DOI: 10.1186/1471-2148-10-308
Evolutionary history of the poly(ADP-ribose) polymerase gene family in eukaryotes
Abstract
Background: The poly(ADP-ribose) polymerase (PARP) superfamily was originally identified as enzymes that catalyze the attachment of ADP-ribose subunits to target proteins using NAD+ as a substrate. The family is characterized by the catalytic site, termed the PARP signature. While these proteins can be found in a range of eukaryotes, they have been best studied in mammals. In these organisms, PARPs have key functions in DNA repair, genome integrity and epigenetic regulation. More recently it has been found that proteins within the PARP superfamily have altered catalytic sites, and have mono(ADP-ribose) transferase (mART) activity or are enzymatically inactive. These findings suggest that the PARP signature has a broader range of functions that initially predicted. In this study, we investigate the evolutionary history of PARP genes across the eukaryotes.
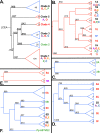
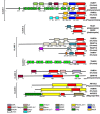
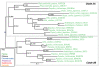
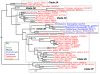
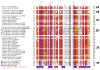
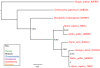
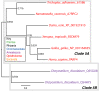
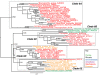
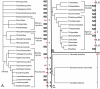
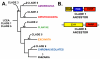
Results: We identified in silico 236 PARP proteins from 77 species across five of the six eukaryotic supergroups. We performed extensive phylogenetic analyses of the identified PARPs. They are found in all eukaryotic supergroups for which sequence is available, but some individual lineages within supergroups have independently lost these genes. The PARP superfamily can be subdivided into six clades. Two of these clades were likely found in the last common eukaryotic ancestor. In addition, we have identified PARPs in organisms in which they have not previously been described.
Conclusions: Three main conclusions can be drawn from our study. First, the broad distribution and pattern of representation of PARP genes indicates that the ancestor of all extant eukaryotes encoded proteins of this type. Second, the ancestral PARP proteins had different functions and activities. One of these proteins was similar to human PARP1 and likely functioned in DNA damage response. The second of the ancestral PARPs had already evolved differences in its catalytic domain that suggest that these proteins may not have possessed poly(ADP-ribosyl)ation activity. Third, the diversity of the PARP superfamily is larger than previously documented, suggesting as more eukaryotic genomes become available, this gene family will grow in both number and type.
Figures


References
-
- Fujimura S, Hasegawa S, Shimizu Y, Sugimura T. Polymerization of the adenosine 5'-diphosphate-ribose moiety of nicotinamide-adenine dinucleotide by nuclear enzyme. I. Enzymatic reactions. Biochim Biophys Acta. 1967;145(247-259) - PubMed
-
- Chambon P, Weil JD, Doly J, Strosser MT, Mandel P. On the formation of a novel adenylic compound by enzymatic extracts of liver nuclei. Biochem Biophys Res Commun. 1966;25:638–643. doi: 10.1016/0006-291X(66)90502-X. - DOI
-
- Nishizuka Y, Ueda K, Nakazawa K, Hayaishi O. Studies on the polymer of adenosine diphosphate ribose. I. Enzymic formation from nicotinamide adenine dinuclotide in mammalian nuclei. J Biol Chem. 1967;242(13):3164–3171. - PubMed
-
- Doly J, Petek F. Etude de la structure d'un compose "poly(ADP-ribose" synthetise par des extraits nucleaires de foie de poulet. CR Hebd Scanc Acad Sci Ser D Sci Nat. 1966;263:1341–1344.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

