Phosphorylation-dependent mineral-type specificity for apatite-binding peptide sequences
- PMID: 20943264
- PMCID: PMC2976791
- DOI: 10.1016/j.biomaterials.2010.08.064
Phosphorylation-dependent mineral-type specificity for apatite-binding peptide sequences
Abstract
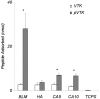
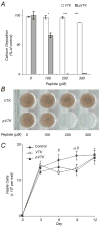
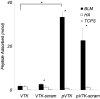
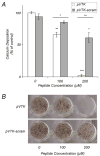
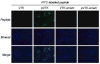
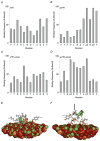
Apatite-binding peptides discovered by phage display provide an alternative design method for creating functional biomaterials for bone and tooth tissue repair. A limitation of this approach is the absence of display peptide phosphorylation--a post-translational modification important to mineral-binding proteins. To refine the material specificity of a recently identified apatite-binding peptide, and to determine critical design parameters (net charge, charge distribution, amino acid sequence and composition) controlling peptide affinity for mineral, we investigated the effects of phosphorylation and sequence scrambling on peptide adsorption to four different apatites (bone-like mineral, and three types of apatite containing initially 0, 5.6 and 10.5% carbonate). Phosphorylation of the VTKHLNQISQSY peptide (VTK peptide) led to a 10-fold increase in peptide adsorption (compared to nonphosphorylated peptide) to bone-like mineral, and a 2-fold increase in adsorption to the carbonated apatite, but there was no effect of phosphorylation on peptide affinity to pure hydroxyapatite (without carbonate). Sequence scrambling of the nonphosphorylated VTK peptide enhanced its specificity for the bone-like mineral, but scrambled phosphorylated VTK peptide (pVTK) did not significantly alter mineral-binding suggesting that despite the importance of sequence order and/or charge distribution to mineral-binding, the enhanced binding after phosphorylation exceeds any further enhancement by altered sequence order. Osteoblast culture mineralization was dose-dependently inhibited by pVTK and to a significantly lesser extent by scrambled pVTK, while the nonphosphorylated and scrambled forms had no effect, indicating that inhibition of osteoblast mineralization is dependent on both peptide sequence and charge. Computational modeling of peptide-mineral interactions indicated a favorable change in binding energy upon phosphorylation that was unaffected by scrambling. In conclusion, phosphorylation of serine residues increases peptide specificity for bone-like mineral, whose adsorption is determined primarily by sequence composition and net charge as opposed to sequence order. However, sequence order in addition to net charge modulates the mineralization of osteoblast cultures. The ability of such peptides to inhibit mineralization has potential utility in the management of pathologic calcification.
Copyright © 2010 Elsevier Ltd. All rights reserved.
Figures
References
-
- Shin H, Jo S, Mikos AG. Biomimetic materials for tissue engineering. Biomaterials. 2003;24(24):4353–4364. - PubMed
-
- Hubbell JA. Bioactive biomaterials. Curr Opin Biotechnol. 1999;10(2):123–129. - PubMed
-
- Geesink RGT. Osteoconductive coatings for total joint arthroplasty. Clin Orthop Relat Res. 2002;395:53–65. - PubMed
-
- Alsberg E, Hill EE, Mooney DJ. Craniofacial Tissue Engineering. Crit Rev Oral Biol & Med. 2001;12(1):64–75. - PubMed
-
- Kokubo T. Apatite formation on organic polymers by a biomimetic process. Eur J Solid State Inorg Chem. 1995;32(7-8):819–827.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

