Searching for a photocycle of the cryptochrome photoreceptors
- PMID: 20943427
- PMCID: PMC2972227
- DOI: 10.1016/j.pbi.2010.09.005
Searching for a photocycle of the cryptochrome photoreceptors
Abstract
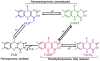
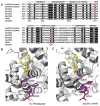
The initial photochemistry of plant cryptochromes has been extensively investigated in recent years. It is hypothesized that cryptochrome photoexcitation involves a Trp-triad-dependent photoreduction. According to this hypothesis, cryptochromes in the resting state contain oxidized FAD; light triggers a sequential electron transfer from three tryptophan residues to reduce FAD to a neutral semiquinone (FADH*); FADH* is the presumed signaling state and it is re-oxidized to complete the photocycle. However, this photoreduction hypothesis is currently under debate. An alternative model argues that the initial photochemistry of cryptochromes involves a photolyase-like cyclic electron shuttle without a bona fide redox reaction mediated by the Trp-triad residues, leading to conformational changes, signal propagation, and physiological responses.
Copyright © 2010 Elsevier Ltd. All rights reserved.
Figures


References
-
- Cashmore AR. Cryptochromes: enabling plants and animals to determine circadian time. Cell. 2003;114:537–543. - PubMed
-
- Sancar A. Structure and function of DNA photolyase and cryptochrome blue-light photoreceptors. Chem Rev. 2003;103:2203–2237. - PubMed
-
- Lin C, Shalitin D. Cryptochrome Structure and Signal transduction. Annu Rev Plant Biol. 2003;54:469–496. - PubMed
-
- Zhu H, Yuan Q, Briscoe AD, Froy O, Casselman A, Reppert SM. The two CRYs of the butterfly. Curr Biol. 2005;15:R953–954. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

