Calcineurin signaling regulates human islet {beta}-cell survival
- PMID: 20943662
- PMCID: PMC3000987
- DOI: 10.1074/jbc.M110.154955
Calcineurin signaling regulates human islet {beta}-cell survival
Abstract
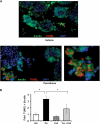
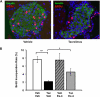
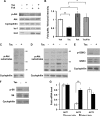
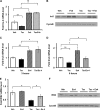
The calcium-regulated phosphatase calcineurin intersects with both calcium and cAMP-mediated signaling pathways in the pancreatic β-cell. Pharmacologic calcineurin inhibition, necessary to prevent rejection in the setting of organ transplantation, is associated with post-transplant β-cell failure. We sought to determine the effect of calcineurin inhibition on β-cell replication and survival in rodents and in isolated human islets. Further, we assessed whether the GLP-1 receptor agonist and cAMP stimulus, exendin-4 (Ex-4), could rescue β-cell replication and survival following calcineurin inhibition. Following treatment with the calcineurin inhibitor tacrolimus, human β-cell apoptosis was significantly increased. Although we detected no human β-cell replication, tacrolimus significantly decreased rodent β-cell replication. Ex-4 nearly normalized both human β-cell survival and rodent β-cell replication when co-administered with tacrolimus. We found that tacrolimus decreased Akt phosphorylation, suggesting that calcineurin could regulate replication and survival via the PI3K/Akt pathway. We identify insulin receptor substrate-2 (Irs2), a known cAMP-responsive element-binding protein target and upstream regulator of the PI3K/Akt pathway, as a novel calcineurin target in β-cells. Irs2 mRNA and protein are decreased by calcineurin inhibition in both rodent and human islets. The effect of calcineurin on Irs2 expression is mediated at least in part through the nuclear factor of activated T-cells (NFAT), as NFAT occupied the Irs2 promoter in a calcineurin-sensitive manner. Ex-4 restored Irs2 expression in tacrolimus-treated rodent and human islets nearly to baseline. These findings reveal calcineurin as a regulator of human β-cell survival in part through regulation of Irs2, with implications for the pathogenesis and treatment of diabetes following organ transplantation.
Figures

References
-
- Cosio F. G., Kudva Y., van der Velde M., Larson T. S., Textor S. C., Griffin M. D., Stegall M. D. (2005) Kidney Int. 67, 2415–2421 - PubMed
-
- Kasiske B. L., Snyder J. J., Gilbertson D., Matas A. J. (2003) Am. J. Transplant. 3, 178–185 - PubMed
-
- Baid S., Cosimi A. B., Farrell M. L., Schoenfeld D. A., Feng S., Chung R. T., Tolkoff-Rubin N., Pascual M. (2001) Transplantation 72, 1066–1072 - PubMed
-
- Bonner-Weir S. (2000) Endocrinology 141, 1926–1929 - PubMed
-
- Davidson J., Wilkinson A., Dantal J., Dotta F., Haller H., Hernández D., Kasiske B. L., Kiberd B., Krentz A., Legendre C., Marchetti P., Markell M., van der Woude F. J., Wheeler D. C. (2003) Transplantation 75, SS3–S24 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

