Chemical reactivities of cysteine substitutions in subunit a of ATP synthase define residues gating H+ transport from each side of the membrane
- PMID: 20943664
- PMCID: PMC3000962
- DOI: 10.1074/jbc.M110.175844
Chemical reactivities of cysteine substitutions in subunit a of ATP synthase define residues gating H+ transport from each side of the membrane
Abstract
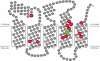
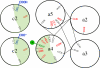
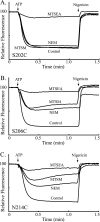
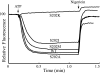
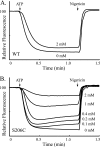
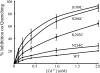
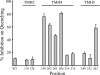
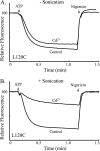
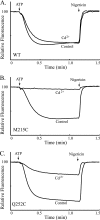
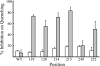
Subunit a plays a key role in coupling H(+) transport to rotations of the subunit c-ring in F(1)F(o) ATP synthase. In Escherichia coli, H(+) binding and release occur at Asp-61 in the middle of the second transmembrane helix (TMH) of F(o) subunit c. Based upon the Ag(+) sensitivity of Cys substituted into subunit a, H(+) are thought to reach Asp-61 via aqueous pathways mapping to surfaces of TMH 2-5. In this study we have extended characterization of the most Ag(+)-sensitive residues in subunit a with cysteine reactive methanethiosulfonate (MTS) reagents and Cd(2+). The effect of these reagents on ATPase-coupled H(+) transport was measured using inside-out membrane vesicles. Cd(2+) inhibited the activity of all Ag(+)-sensitive Cys on the cytoplasmic side of the TMHs, and three of these substitutions were also sensitive to inhibition by MTS reagents. On the other hand, Cd(2+) did not inhibit the activities of substitutions at residues 119 and 120 on the periplasmic side of TMH2, and residues 214 and 215 in TMH4 and 252 in TMH5 at the center of the membrane. When inside-out membrane vesicles from each of these substitutions were sonicated during Cd(2+) treatment to expose the periplasmic surface, the ATPase-coupled H(+) transport activity was strongly inhibited. The periplasmic access to N214C and Q252C, and their positioning in the protein at the a-c interface, is consistent with previous proposals that these residues may be involved in gating H(+) access from the periplasmic half-channel to Asp-61 during the protonation step.
Figures
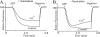
Similar articles
-
Obstruction of transmembrane helical movements in subunit a blocks proton pumping by F1Fo ATP synthase.J Biol Chem. 2013 Aug 30;288(35):25535-25541. doi: 10.1074/jbc.M113.496794. Epub 2013 Jul 17. J Biol Chem. 2013. PMID: 23864659 Free PMC article.
-
Aqueous access pathways in ATP synthase subunit a. Reactivity of cysteine substituted into transmembrane helices 1, 3, and 5.J Biol Chem. 2007 Mar 23;282(12):9001-7. doi: 10.1074/jbc.M610848200. Epub 2007 Jan 18. J Biol Chem. 2007. PMID: 17234633
-
Aqueous accessibility to the transmembrane regions of subunit c of the Escherichia coli F1F0 ATP synthase.J Biol Chem. 2009 Aug 28;284(35):23243-50. doi: 10.1074/jbc.M109.002501. Epub 2009 Jun 19. J Biol Chem. 2009. PMID: 19542218 Free PMC article.
-
Half channels mediating H(+) transport and the mechanism of gating in the Fo sector of Escherichia coli F1Fo ATP synthase.Biochim Biophys Acta. 2014 Jul;1837(7):1063-8. doi: 10.1016/j.bbabio.2014.03.005. Epub 2014 Mar 17. Biochim Biophys Acta. 2014. PMID: 24650630 Review.
-
Mechanics of coupling proton movements to c-ring rotation in ATP synthase.FEBS Lett. 2003 Nov 27;555(1):29-34. doi: 10.1016/s0014-5793(03)01101-3. FEBS Lett. 2003. PMID: 14630314 Review.
Cited by
-
Alkaliphilic Bacteria with Impact on Industrial Applications, Concepts of Early Life Forms, and Bioenergetics of ATP Synthesis.Front Bioeng Biotechnol. 2015 Jun 3;3:75. doi: 10.3389/fbioe.2015.00075. eCollection 2015. Front Bioeng Biotechnol. 2015. PMID: 26090360 Free PMC article. Review.
-
Structure of ATP synthase from Paracoccus denitrificans determined by X-ray crystallography at 4.0 Å resolution.Proc Natl Acad Sci U S A. 2015 Oct 27;112(43):13231-6. doi: 10.1073/pnas.1517542112. Epub 2015 Oct 12. Proc Natl Acad Sci U S A. 2015. PMID: 26460036 Free PMC article.
-
Resolving the negative potential side (n-side) water-accessible proton pathway of F-type ATP synthase by molecular dynamics simulations.J Biol Chem. 2012 Oct 19;287(43):36536-43. doi: 10.1074/jbc.M112.398396. Epub 2012 Aug 31. J Biol Chem. 2012. PMID: 22942277 Free PMC article.
-
Obstruction of transmembrane helical movements in subunit a blocks proton pumping by F1Fo ATP synthase.J Biol Chem. 2013 Aug 30;288(35):25535-25541. doi: 10.1074/jbc.M113.496794. Epub 2013 Jul 17. J Biol Chem. 2013. PMID: 23864659 Free PMC article.
-
High-resolution structure and mechanism of an F/V-hybrid rotor ring in a Na⁺-coupled ATP synthase.Nat Commun. 2014 Nov 10;5:5286. doi: 10.1038/ncomms6286. Nat Commun. 2014. PMID: 25381992 Free PMC article.
References
-
- Yoshida M., Muneyuki E., Hisabori T. (2001) Nat. Rev. Mol. Biol. 2, 669–677 - PubMed
-
- Capaldi R. A., Aggeler R. (2002) Trends Biochem. Sci. 27, 154–160 - PubMed
-
- von Ballmoos C., Wiedenmann A., Dimroth P. (2009) Annu. Rev. Biochem. 78, 649–672 - PubMed
-
- Senior A. E. (1988) Physiol. Rev. 68, 177–231 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

