Efficacy model for antibody-mediated pre-erythrocytic malaria vaccines
- PMID: 20943696
- PMCID: PMC3061132
- DOI: 10.1098/rspb.2010.1697
Efficacy model for antibody-mediated pre-erythrocytic malaria vaccines
Abstract
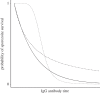
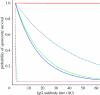
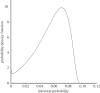
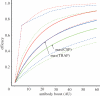
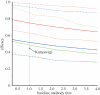
Antibodies to the pre-erythrocytic antigens, circumsporozoite protein (CSP), thrombospondin-related adhesive protein (TRAP) and liver-stage antigen 1, have been measured in field studies of semi-immune adults and shown to correlate with protection from Plasmodium falciparum infection. A mathematical model is formulated to estimate the probability of sporozoite infection as a function of antibody titres to multiple pre-erythrocytic antigens. The variation in antibody titres from field data was used to estimate the relationship between the probability of P. falciparum infection per infectious mosquito bite and antibody titre. Using this relationship, we predict the effect of vaccinations that boost baseline CSP or TRAP antibody titres. Assuming the estimated relationship applies to vaccine-induced antibody titres, then single-component CSP or TRAP antibody-mediated pre-erythrocytic vaccines are likely to provide partial protection from infection, with vaccine efficacy of approximately 50 per cent depending on the magnitude of the vaccine-induced boost to antibody titres. It is possible that the addition of a TRAP component to a CSP-based vaccine such as RTS,S would provide an increase in infection-blocking efficacy of approximately 25 per cent should the problem of immunological interference between antigens be overcome.
Figures
References
-
- Snow R. W., Guerra C. A., Noor A. M., Myint H. Y., Hay S. I. 2005. The global distribution of clinical episodes of Plasmodium falciparum malaria. Nature 434, 214–21710.1038/nature03342 (doi:10.1038/nature03342) - DOI - DOI - PMC - PubMed
-
- Meis J., et al. 1986. Fine-structure of the malaria parasite Plasmodium falciparum in human hepatocytes in vitro. Cell Tissue Res. 244, 345–350 - PubMed
-
- Doolan D. L., Dobano C., Baird J. K. 2009. Acquired immunity to malaria. Clin. Microbiol. Rev. 22, 13–3610.1128/CMR.00025-08 (doi:10.1128/CMR.00025-08) - DOI - DOI - PMC - PubMed
-
- Alonso P. L., et al. 2004. Efficacy of the RTS,S/AS02A vaccine against Plasmodium falciparum infection and disease in young African children: randomised controlled trial. Lancet 364, 1411–142010.1016/S0140-6736(04)17223-1 (doi:10.1016/S0140-6736(04)17223-1) - DOI - DOI - PubMed
-
- Bejon P., et al. 2008. Efficacy of RTS,S/AS01E vaccine against malaria in children 5 to 17 months of age. New Engl. J. Med. 359, 2521–253210.1056/NEJMoa0807381 (doi:10.1056/NEJMoa0807381) - DOI - DOI - PMC - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
